Many forms of cancer fail to respond to chemotherapy by acquiring multidrug resistance (MDR), to which has been attributed the failure of treatment in over 90% of patients with metastatic cancer. Although MDR can have several causes, one major form of resistance to chemotherapy has been correlated with the presence of MDR "pumps" that actively transport drugs out of the cell. The most prevalent of these MDR transporters is P-glycoprotein (P-gp), a member of the adenosine triphosphate (ATP)-binding cassette (ABC) superfamily. P-glycoprotein (P-gp) detoxifies cells by exporting hundreds of chemically unrelated toxins but has been implicated in multidrug resistance in the treatment of cancers. Most P-gp substrates are hydrophobic and partition into the lipid bilayer. Thus, P-gp has been likened to a molecular "hydrophobic vacuum cleaner", extracting substrates from the membrane and expelling them to promote multidrug resistance. Although the structures of bacterial ABC importers and exporters have been established, P-gp has only been characterized at low resolution by electron microscopy. Substrate promiscuity is a hallmark of P-gp activity, thus a structural description of poly-specific drug-binding is important for the rational design of anticancer drugs and MDR inhibitors.
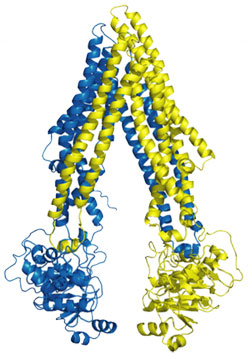
Using data collected at SSRL Beam Lines 9-2, 11-1, and at ALS and APS, a research team led by Geoffrey Chang from Scripps Research Institute determined the three dimensional structure of P-gp and the inhibitor bound forms. The x-ray structure of apo P-gp at 3.8 angstroms reveals an internal cavity of 6000 angstroms cubed with a 30 angstrom separation of the two nucleotide-binding domains (Fig 1). The P-gp structures with cyclic peptide inhibitors demonstrate distinct drug-binding sites in the internal cavity capable of stereo selectivity that is based on hydrophobic and aromatic interactions. Apo and drug-bound P-gp structures have portals open to the cytoplasm and the inner leaflet of the lipid bilayer for drug entry. The structure of P-gp represents a nucleotide-free inward-facing conformation arranged as two "halves" with pseudo two-fold molecular symmetry spanning 136 Å perpendicular to and 70 Å in the plane of the bilayer.
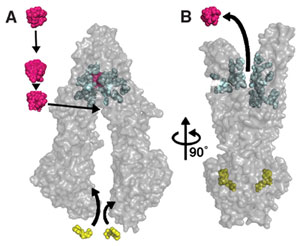
The inward-facing structure does not allow substrate access from the outer membrane leaflet nor the extracellular space and this conformation could represent the molecule in a pre-transport state, because it is competent to bind drug. This conformation likely represents an active state of P-gp because protein recovered from crystals had significant drug-stimulated ATPase activity. During the catalytic cycle, binding of ATP, stimulated by substrate, likely causes a dimerization in the NBDs, which produces large structural changes resulting in an outward-facing conformation similar that is nucleotide-bound. ATP hydrolysis likely disrupts NBD dimerization and resets the system back to inward facing and reinitiates the transport cycle.
his work was supported by grants from the Army (W81XWH-05-1-0316), NIH (GM61905, GM078914, GM073197), the Beckman Foundation, the Skaggs Chemical Biology Foundation, Jasper L. and Jack Denton Wilson Foundation, the Southwest Cancer and Treatment Center, and the Norton B. Gilula Fellowship.
- D. B. Longley, P. G. Johnston, J. Pathol. 205, 275 (2005).
- F. J. Sharom, Pharmacogenomics 9, 105 (2008).
- K. Hollenstein, D. C. Frei, K. P. Locher, Nature 446, 213 (2007).
- H. W. Pinkett, A. T. Lee, P. Lum, K. P. Locher, D. C. Rees, Science 315, 373 (2007).
- R. J. P. Dawson, K. P. Locher, Nature 443, 180 (2006).
- A. Ward, C. L. Reyes, J. Yu, C. B. Roth, G. Chang, Proc. Natl. Acad. Sci. U.S.A. 104, 19005 (2007)./li>
Stephen G. Aller, Jodie Yu, Andrew Ward, Yue Weng, Srinivas Chittaboina, Rupeng Zhuo, Patina M. Harrell, Yenphuong T. Trinh, Qinghai Zhang, Ina L. Urbatsch, Geoffrey Chang (2009) Structure of P-Glycoprotein Reveals a Molecular Basis for Poly-specific Drug Binding. Science, 323, 1718-1722.

