Hydrogenases catalyze the stoichiometrically simple (H2 ↔ 2 H+ + 2 e-), but energetically difficult dihydrogen uptake/evolution reactions.1 The FeFe-hydrogenases are of great interest since they can catalyze both the forward and the reverse reactions. Under optimal conditions a single molecule of FeFe-hydrogenase can produce approximately 6000 molecules of hydrogen within a second. This translates into a theoretical capacity for refueling the hydrogen tank of the Space Shuttle within 30 minutes. Thus, hydrogenases are considered desirable biological targets for hydrogen-based energy production and storage technologies.
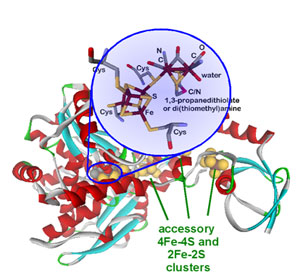
Protein crystallography revealed the molecular structure of the enzyme (Figure 1),2,3 which is composed of a single amino acid chain with multiple iron-sulfur clusters. The catalytically active cluster (H-cluster) shows a remarkable molecular structure with unique organometallic character. The hydrogenases are the only group of metalloenzymes that contain carbon monoxide and cyanide ligands which are generally toxic to living organisms; however, they are absolutely required for hydrogenase activity. The accessory iron-sulfur clusters are thought to be important in efficient electron transfer to/from the H-cluster.4 Several spectroscopic techniques have already been applied to understand the structure of this unique cluster and due to overlapping spectral features from the accessory iron-sulfur clusters it has been difficult to determine the physico-chemical properties of the H-cluster that are responsible for its high catalytic activity.
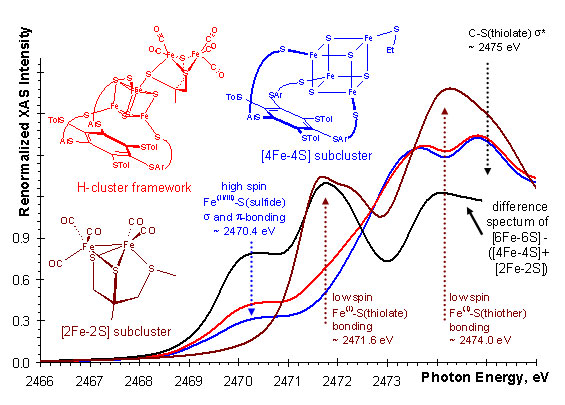
Biomimetic chemistry provides an alternative way to probe the structural properties of the H-cluster that is not be possible from direct experiments on the protein samples. We have utilized structurally analogous iron-sulfur clusters5 to gain insight into the electronic structure of the H-cluster by X-ray absorption spectroscopy (XAS). In the 2-5 keV energy range (accessible at beamline 6-2 of SSRL), spectral features observed below the ionization threshold of the sulfur 1s core electrons are directly related to the nature and strength of chemical bonds between sulfur atoms and transition metal ions.6 The energy positions and intensities of these features are characteristic of the type and strength of specific metal-sulfur interactions, respectively. The assignment of spectral features is commonly aided by using structurally well-defined model complexes. Spectroscopically calibrated density functional theory (DFT) calculations7 aid in bridging the gap between the model complexes and the ill-defined protein bound active sites.
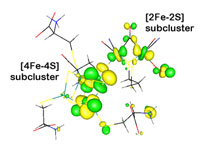
Using XAS in combination with DFT, the strength of the chemical interaction was probed between the [4Fe-4S] and the [2Fe-2S] subclusters of the H-cluster. By comparing the spectra of each subcluster and the H-cluster framework, we found evidence (Figure 2) for considerable electron delocalization between the subclusters upon formation of the iron-bridging thiolate-iron bond. This suggest that the H-cluster is an electronically inseparable [6Fe-6S] cluster. Thus, the redox chemistry and substrate activation is determined by both subclusters together and not just the [2Fe-2S] subcluster which has been the focus of much past research. DFT calculations on separate and the combined subclusters also show this delocalization by redox active molecular orbitals that span the entire 6Fe-framework (Figure 3).
Recently a heterologous expression system for FeFe-hydrogenases was developed, which opened up the possibility for direct spectroscopic studies on the metalloenzyme. An active version of the FeFe-hydrogenase can be expressed in the absence of the accessory iron-sulfur clusters, thus reducing the background of iron and sulfur in the XAS experiment. Further spectroscopic and computational studies on biomimetic complexes, metalloprotein samples, and in silico chemical models have the potential to assist in the design of inexpensive iron/sulfur/carbonyl-based catalytic systems for hydrogen production and conversion to electronic energy which could replace the expensive noble metal-based systems.
- Adams, M. W. W. Biochim. Biophys. Acta 1990, 1020, 115.
- Peters, J. W.; Lanzilotta, W. N.; Lemon, B. J.; Seefeldt, L. C. Science 1998, 282, 1853-1858.
- Nicolet, Y.; Piras, C.; Legrand, P.; Hatchikian, C. E.; Fontecilla-Camps, J. C. Struct. Fold Des. 1999, 7, 13-23.
- Nicolet, Y.; Lemon, B. J.; Fontecilla-Camps, J. C.; Peters, J. W. Trends Biochem. Sci. 2000, 25, 138-143.
- Tard, C.; Liu, X. M.; Ibrahim, S. K.; Bruschi, M.; De Gioia, L.; Davies, S. C.; Yang, X.; Wang, L. S.; Sawers, G.; Pickett, C. J. Nature 2005, 433, 610-613.
- Solomon, E. I.; Hedman, B.; Hodgson, K. O.; Dey, A.; Szilagyi, R. K. Coord. Chem. Rev. 2005, 249, 97-129.
- Szilagyi, R. K.; Winslow, M. A. J. Comput. Chem. 2006, 27, 1385-1397.
- Posewitz, M. C.; King, P. W.; Smolinski, S. L.; Smith, R. D.; Ginley, A. R.; Ghirardi, M. L.; Seibert, M. Biochem. Soc. Trans. 2005, 33, 102-104.
Schwab, D. E.; Tard, C.; Brecht, E.; Peters, J. W.; Pickett, C. J.; Szilagyi, R. K. Chem. Commun. 2006, 3696-3698.

