Cadmium is known to be extremely toxic to mammals, and is generally viewed alongside mercury an environmental problem and toxic element that is not used by nature in any way. We have reported the characterization of a previously unknown metalloenzyme from the marine diatom Thalassiosira weissflogii that specifically uses cadmium to achieve its biological function. This work shows that we need to revise our opinion of cadmium - it appears that it is not only used biologically, but may play a vital role in the global carbon cycle.
Carbonic anhydrases catalyze the inter-conversion of carbonic acid and carbon dioxide
HCO3- + H+ <-------> CO2 + H2O
They are among the fastest enzymes known, with turnover numbers close to one million per second. Photosynthesis in green plants can use only molecular carbon dioxide, and not carbonic acid, and carbonic anhydrase thus represents the first step in the process of photosynthesis. In marine microalgae (e.g. diatoms), carbonic acid is taken into the cell by a carbonic acid pump, converted to CO2 by carbonic anhydrase, and then subsequently fed into the Calvin cycle by ribulose bis-phosphate carboxylase, which uses molecular CO2. All previously characterized carbonic anhydrases incorporate an atom of zinc into the active site, and these are divided into three categories - referred to as a, b, g carbonic anhydrases. The a-carbonic anhydrases are by far the best studied, being found in animals (including mammals). They share several highly conserved sequence elements, and contain zinc coordinated by three histidines and (probably) a hydroxyl (1). The majority of the higher plant isoforms make up the second class, and
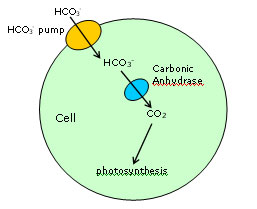
are referred to as b-carbonic anhydrases. These contain no sequence homology to the a-carbonic anhydrases, and contain zinc ligated by two cysteine and one histidine, plus an activated water or hydroxyl (2,3). A closely related coordination is observed in the b-carbonic anhydrases of the red alga Porphyridium purpureum (4). The prototype for the third (g) class has been reported for the bacterium Methanosarcina thermophila (5,6), and shows a zinc coordination resembling the a-carbonic anhydrases. We have previously reported that the diatom T. weissflogii when grown in the presence of zinc produces zinc-containing carbonic anhydrase which has an active site structure that is very similar to the a-carbonic anhydrases, but with no sequence homology (7). We further proposed that this should be designated as a fourth class the d-carbonic anhydrases, and concluded that the active site structure was a striking example of convergent evolution at the molecular level (7).
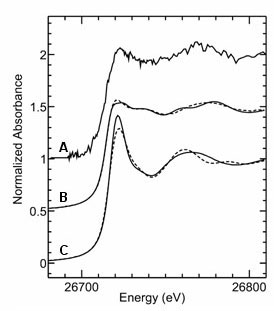
It is well established that the surface waters of the oceans, in which microalgae such as diatoms flourish, are extremely low in zinc - between 2 and 50 pico-molar. T. wiessflogii contains genes for two discrete carbonic anhydrases. This, together with the observation that adding cadmium allows the diatom to grow (8), caused us to search for a specific cadmium enzyme. Figure 3 shows the Cd K-edge spectra, collected on SSRL's beamline 7-3, of the Cd carbonic anhydrase isolated from the diatom, and several different model species. The concentration of the enzyme was only 7 µM, and the data presented was the sum of 59 individual 25 minute scans. Comparison of the near-edge spectra of Figure 3, allows us to formulate some conclusions about the nature of the active site. The Cd-carbonic anhydrase spectrum clearly resembles, but is not identical to, those of the tetrahedral models with a lot of thiolate coordination. It seems very likely that the metal contains an activated water or hydroxyl ligand, and a structure homologous to the higher plant b-carbonic anhydrases seems plausible, but more definitive conclusions must await data from more concentrated samples of the enzyme. The enzyme amino acid sequence is distinct from all other carbonic anhydrases, and therefore represents yet another discrete class, which we denote as
z-carbonic anhydrase.
Despite their microscopic size, marine phytoplankton are very numerous, and make up a significant fraction of the world's plants. They are thus responsible for a significant fraction of the cycling of atmospheric carbon dioxide through photosynthesis. Cadmium is needed for this, at least in diatoms but probably in other marine micro-algae too, so it may be that cadmium, rather than being an environmentally detrimental element, is environmentally essential in the global sense.
- Fisher, Z.; Hernandez Prada, J. A.; Tu, C.; Duda, D.; Yoshioka, C.; An, H.; Govindasamy, L.; Silverman, D. N.; McKenna, R. Biochemistry 2005, 44, 1097-1105 (and references therein).
- Bracey, M. H.; Christiansen, J.; Tovar, P.; Cramer, S. P.; Bartlet, S. G. Biochemistry, 1994, 33, 13126-13131.
- Mitsuhashi, S.; Mizushima, T.; Yamashita, E.; Yamamoto, M.; Kumasaka, T.; Moriyama, H.; Ueki, T.; Miyachi, S.; Tsukihara, T. J. Biol. Chem. 2000, 275, 5521-526.
- Kisker, C.; Schindelin, H.; Alber, B. E.; Ferry, J. G.; Rees, D. C. EMBO J. 1996, 15, 2323-2330.
- Iverson, T. M.; Alber, B. E.; Kisker, C.; Ferry, J. G.; Rees, C. D. Biochemistry 2000, 39, 9222-9231.
- Cox, E. H.; McLendon, G. L.; Morel, F. M. M.; Prince, R. C.; Pickering, I. J.; George G. N. Biochemistry 2000, 39, 12128-12130.
- Morel F. F. M.; Price, N. M. Science, 2003, 300, 944-947 (and references therein).
Kimber, M. S.; Pai, E. F. EMBO J. 2000, 19, 1407-1418.

