In the well-known Greek legend the touch of King Midas would convert anything to metallic gold. Recently, a team working at SSRL lead by Professor Jorge Gardea-Torresdey from the University of Texas at El Paso have shown that ordinary alfalfa plants can accumulate very small particles (nanoparticles) of metallic gold (1). The best-known materials that contain nanoparticles of metallic gold are gold colloids. These lack the familiar metallic luster, but show bright colors which range from red, violet or blue, depending upon the size of the nanoparticles (2,3). Colloidal gold has traditionally been used to color materials such as glass (e.g. gold ruby glass and cranberry glass) and enamels (e.g. famille rose enamels) since the 16th century. The earliest report of a colloidal gold preparation may be in the Bible. The book of Exodus reports that Moses destroyed the golden calf in a manner that may have resulted in an aqueous (water-based) gold colloid, which he then forced the Israelites to drink. In modern times gold colloids are imbibed for a variety of ailments (despite little or no evidence of any health-related benefits), but the most important applications may be in the field of nano-technology (see 1, and refs therein).
Gold salts are readily reduced to elemental gold, and chemical methods for making gold nanoparticles have been known for a long time, but only in recent years has accurate control of nanoparticle size been achieved (4, see also discussion in (1). Unfortunately, many of the new methods have proven to be cumbersome or give rise to toxic side-products, and a need to develop better methods is indicated. The use of biomaterials provides a possible alternative, and Professor Gardea-Torresdey and co-workers chose alfalfa as a model system. Plants were germinated and grown on gold-rich agar media and plant tissue samples were analyzed using Au L-edge X-ray absorption spectroscopy (XAS) on SSRL’s Beam Line 7-3, and at UT-Austin using high resolution transmission electron microscopy lead by Professor Miguel Jose Yacaman.
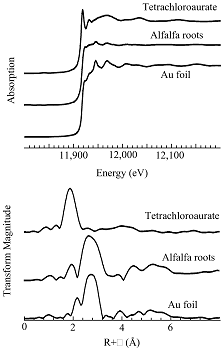
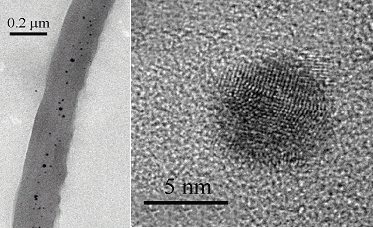
The near-edge spectra (e.g. figure 1, top) suggests that the gold in the plant samples and the agar growth media is present as elemental gold [Au(0)], and that alfalfa therefore has the ability to take up Au(0) from the media, and transport it throughout the plant. Quantitative analysis of the EXAFS (figure 1, bottom) indicates that gold is present as gold particles. As expected from the FCC crystal structure, the Au-Au bond of the metallic gold foil has a distance of 2.86 Å and a coordination number of 12, and the plant samples have shown the same Au-Au bond-length, but with reduced apparent coordination number (~6), presumably as a result of disorder in the gold structure arising from small particle sizes. Figure 2 shows low (left) and high resolution (right) transmission electron micrographs of gold particles in alfalfa. The dark dots in low the magnification image correspond to the gold nanoparticles. These data suggest that the nucleation and transport of the particles inside the plants occurs in preferential zones. The high-resolution images of the gold nanoparticles (figure 2, right) clearly show regular structure indicative of a crystalline state.
This biologically-based method of making metallic gold nanoparticles is both cost-effective and environmentally friendly. It could be adapted to produce gold nanoparticles for nano-electronic or nano-optical devices. Future studies will involve the full characterization and the development of methods to extract the nanoparticles from the plants.
The synchrotron aspects of this project were enabled through the DOE/SSRL Gateway program and the beam time provided at SSRL. SSRL is a national synchrotron user facility operated by the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL Gateway program, also supported by DOE-BES, is a joint cooperative research and training effort by SSRL and University of Texas at El Paso to engage young Mexican and Mexican-American students in frontier scientific research using advanced facilities.
- Gardea-Torresdey, J. L.; Parsons, J. G.; Gomez, E.; Peralta-Videa, J.; Troiani, H. E.; Santiago, P.; Yacaman, M. J.; Nano Lett.; (Communication); 2002; 2(4); 397-401.
- Turkevich J., Colloidal Gold Part I: Historical and Preparative Aspects, Morphology and Structure, 1985. Gold Bulletin, 18, 86-91.
- Turkevich J., Colloidal Gold Part II: Colour, Coagulation, Adhesion, Alloying and Catalytic Properties, 1985. Gold Bulletin, 18, 125-131.
- Whyman, R. Gold nanoparticles. A renaissance in gold chemistry. Gold Bull. (London) (1996), 29(1), 11-15.

