Structural changes leading to disordering of the cation-water arrangement within the pores of zeolite natrolite while exchanging sodium (Na+) with potassium (K+) have been investigated using x-ray diffraction (XRD) and oxygen K-edge x-ray absorption spectroscopy (XAS).
The most fundamental chemical property of zeolites is ion exchange. A detailed understanding of why and how ion exchange occurs is of tremendous importance for many industrial processes ranging from well-known water softening to environmental cleanup such as radionuclide storage.
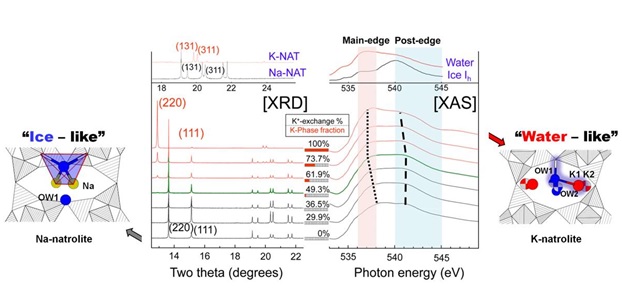
In the case of sodium natrolite, rubidium- or cesium-containing natrolites can only be made if Na+ is first exchanged with K+ [1,2]. This increased reactivity was not understood until a team of researchers from Korea (led by Yongjae Lee from Yonsei University), SSRL (Jun-Sik Lee, Dennis Nordlund, Chi-Chang Kao) and the University of South Carolina (Thomas Vogt) showed conclusively that, when exchanging K+ for Na+, K+ is located near the walls of the pores and not near their center as Na+ in Na-natrolite (Fig. 1).
Concomitantly, the water molecules change positions too: In Na-natrolite water is located near the walls of the pores, whereas it is located near the center of the pores in K-natrolite. This site exchange also changes the character of the water from more “ice-like” in Na-natrolite to more “water-like” in K-natrolite (Fig. 2). This evolution was followed using oxygen K-edge XAS at SSRL’s Beam Line 10-1. In these experiments, a shift in energies and relative intensities indicated a higher degree of disorder in K-natrolite. With the current work, the researchers correlated these structural and dynamic features to the chemical reactivity of ion exchange in natrolite.
The team has also shown in other work [3] that the ion-exchange property itself can be tuned by pressure, enabling non-conventional zeolite applications. The researchers are currently developing unique high-pressure instrumentation at SSRL’s Beam Line 10-2 through the Collaborative Access Program.
This work was supported by the Global Research Laboratory program of the Korean Ministry of Science, ICT, and Future Planning (MSIP). XRD experiments were performed at Pohang Accelerator Laboratory, which is supported in part by the MSIP and POSTECH. XAS experiments were carried out at the SSRL, a Directorate of SLAC and an Office of Science User Facility operated by Stanford University for the U.S. Department of Energy Office of Science.
1. Y. Lee, Y. Lee and D. Seoung, “Natrolite May Not be a “Soda-Stone” Anymore: Structural Study of fully K-, Rb-, and Cs-exchanged Natrolite”, Am. Mineral. 95, 1636 (2010)
2. Y. Lee, Y. Lee and D. Seoung, “Natrolite is Not a "Soda-Stone" Anymore: Structural Study of Alkali (Li+), Alkaline-Earth (Ca2+, Sr2+, Ba2+) and Heavy Metal (Cd2+, Pb2+, Ag+) Cation-exchanged Natrolites”, Am. Mineral. 96, 1718 (2011)
3. Y. Lee, Y. Lee, D. Seoung, J.-H. Im, H. J. Hwang, T. H. Kim, D. Liu, Z. Liu, S. Y. Lee, C.-C. Kao and T. Vogt, “Immobilization of Large, Aliovalent Cations in the Small-Pore Zeolite K-Natrolite by Means of Pressure”, Angew., Chem. Int. Ed. Engl. 51, 4848 (2012)
Y. Lee, J.-S. Lee, C.-C. Kao, J.-H. Yoon, T. Vogt and Y. Lee, “Role of Cation–Water Disorder during Cation Exchange in Small-Pore Zeolite Sodium Natrolite”, J. Phys. Chem. C 117, 16119 (2013), DOI: 10.1021/jp405360s.

