Researchers using X-rays to study a single-atom-thick layer of carbon, called graphene, have learned new information about its atomic bonding and electronic properties when the material is "doped" with nitrogen atoms. They show that synchrotron X-ray techniques can be excellent tools to study and better understand the behavior of doped graphene, which is being eyed for use as a promising contact material in electronic devices due to its many desirable traits, including high conductivity and, most notably, tunable electronic properties.Doping graphene with small amounts of another element, such as nitrogen or boron, turns it into either an "n-type" material (having excess negative charge carriers, i.e., electrons, as in the case of nitrogen) or a "p-type" material (having excess positive charge carriers, i.e., electron vacancies called "holes" as in the case of boron). In this way, doping allows scientists to "tune" its properties, including the types of bonds between the atoms and how charge carriers are distributed. This kind of control is key when developing a material with specific applications in mind. A similar example is the doping of silicon used in silicon-based photovoltaics; indeed, doped graphene is being examined for its potential use as a contact material in solar cells (among its many suitable qualities for such a role is its transparency to visible light, a necessary feature for a solar-cell electrical contact).
The high energy resolution and tunable polarization and energy of synchrotron light enable the detection of chemically distinct species and the identification of bond types – even of sub-percent level dopants – in monolayer graphene. At SSRL Beam Lines 10-1 and 13-2 (and NSLS Beam Line U7A) Schiros and her team employed three soft X-ray techniques to study their graphene samples: X-ray photoelectron spectroscopy (XPS), carbon and nitrogen core-level X-ray absorption spectroscopy (XAS), and carbon core level X-ray emission spectroscopy (XES). Each technique works by taking advantage of one way in which X-rays can interact with a sample; therefore, each provides unique information about that sample.
XPS measures the number and energies of the electrons that escape the surface of a sample when it is illuminated with X-rays, and therefore provides information on the elemental concentration and binding energies, which reflect the local chemical bond environment. XAS provides direct information about the type of bond between the nitrogen and carbon atoms, the orientation of that bond, and the unoccupied molecular orbitals formed between dopant and host atoms. XES provides complementary, atom-specific and symmetry-resolved information about the local occupied electron energy levels near the "Fermi level," which plays a key role in graphene's electronic behavior. In combination with density functional theory (DFT)-based calculations, the suite of complementary X-ray techniques was used to obtain an atom-specific picture of dilutely doped, single-layer graphene, despite the small probability of radiative core hole decay and the weak signal from sub-percent atomic dopant levels. The work produced a clear, microscopic picture of the different bond types and their diverse effects on the work function, carrier concentration, and the local electronic structure in nitrogen-doped graphene films.
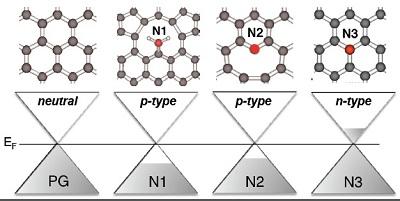
In this work, the researchers discovered that several bond types may be present between carbon and nitrogen atoms, even within the same graphene sheet, and that the different N-bond types have profoundly different macroscopic electronic structure effects on the graphene. With clear correlations between the computed and measured spectroscopic signatures, DFT calculations for extended graphene sheets with 1% of the dopant structures were used to probe changes in the work function for the different N-bond environments and the fractional charge added (withdrawn) per N atom for each dopant. The investigation showed that graphitic nitrogen (N3) bonded to three carbon atoms n-dopes graphene, consistent with the expected picture of substitutional N doping. In contrast, pyridinic (N2) and nitrile-like (with or without H-termination (N1)) dopants, which have two bonds and one bond, respectively, to C in the graphene sheet, occurring at vacancy sites and domain edges, and vacancy defects themselves, produce p-type doping of graphene. In each of the three dopant bond structure motifs, ~0.5 electron per N dopant is added to or withdrawn from the carbon π- network, which is in excellent agreement with scanning tunneling spectroscopy data [1]. These findings were experimentally confirmed by measuring the work function of transferred graphene with various degrees of N2 and N1 dopants relative to pristine graphene at Beam Line 10-1. This understanding, coupled with increasing control over N2/N3 ratios in N-doped graphene, opens new avenues for tailoring the carrier density and electronic properties of graphene at the atomic level.
These findings show that controlling the bonding type of dopant substitutions in graphene is essential when designing properties of graphene as functional component in future devices. This work demonstrates that synchrotron radiation-based spectroscopies provide invaluable, atom-specific tools to determine the resulting electronic properties of different dopant and defect structures in graphene, even at sub-percent dopant levels. Combined with continuing advances in the growth and controlled doping of graphene, and cleaner transfer procedures, the detailed correlation of bond type and electronic structure demonstrated here promises to facilitate the atomic-level control of electronic properties desired for next generation graphene-based devices.
The work was led by Theanne Schiros, a research fellow at the Energy Frontier Research Center at Columbia University, in close collaboration with SSRL scientist Dennis Nordlund. The results were published in the June 29, 2012, online edition of Nano Letters. The paper's co-authors include colleagues at Columbia University as well as the Stanford Synchrotron Radiation Lightsource (SSRL), CNR-Nanoscience Institute (Italy), Sejong University (Korea), the National Institute of Standards and Technology, Stockholm University (Sweden), and Brookhaven National Laboratory.
1. L. Zhao, R. He, K. T. Rim, T. Schiros, K. S. Kim, H. Zhou, C. Gutiérrez, S. P. Chockalingam, C. J. Arguello, L. Pálová, D. Nordlund, M. S. Hybertsen, D. R. Reichman, T. F. Heinz, P. Kim, A. Pinczuk, G.W. Flynn and A. N. Pasupathy, “Visualizing Individual Nitrogen Dopants in Monolayer Graphene”, Science 333, 999 -1003 (2011).
T. Schiros, D. Nordlund, L. Pálová, D. Prezzi, L. Zhao, K. S. Kim, U. Wurstbauer, C. Gutierrez, D. Delongchamp, C. Jaye, D. Fischer, H. Ogasawara, L. G. M. Pettersson, D. R. Reichman, P. Kim, M. S. Hybertsen and A. N. Pasupathy, "Connecting Dopant Bond Type with Electronic Structure in N-Doped Graphene", Nano Lett. 12, 4025 (2012) doi: 10.1021/nl301409h




