The ability to design and control the activities of transition metal catalysts, which are scarce in nature and thus expensive, has been of great importance to the development of economical industrial and energy-saving processes. Over the years several methods have been suggested, especially for processes using platinum, which is the most active metal catalyst for many important reactions, including the reduction of oxygen in fuel cells.
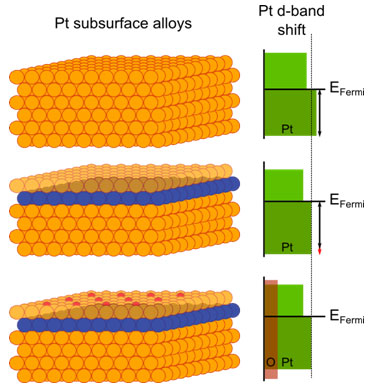
Tuning chemical functionality by implementing a ligand effect – in other words, by changing the atomic nearest neighbor environment – is a conceptually simple way to modify the electronic structure of platinum to achieve enhanced catalytic activity. For example, the authors have shown that embedding nickel and cobalt in the subsurface region of Pt(111) will tune the electronic structure of the Pt catalyst, giving rise to changes in the strength of the bonding between Pt and oxygen and hydrogen adsorbates. The authors used element-specific core level spectroscopy and probed separately both the metal valence states and the density of the adsorbate (oxygen) states. This demonstrated how to modify the electronic configuration of the adsorbate-metal bond and how the resulting effect can translate to improved catalytic activity. This unique probing method differentiates itself from other, more conventional, methods which probe the superimposed electronic structure of the systems.
Changes in the electronic structure of platinum due to nickel and cobalt embedded in the subsurface region is schematically shown in Figure 1. Ligand effect shifts the Pt d-band valence states, moving the center of the d-band further away from the Fermi level. This shift is also correlated to the group number of the transition metal in the periodic table; in other words, the effect induced by cobalt is more pronounced than that induced by nickel. Thus, a trend in catalytic activity is expected. The shift results in a modified degree of interaction between Pt and adsorbate states: a weaker or stronger chemical bond between them.
Correlation of electronic properties to catalytic activity is possible with the help of the unique capabilities of SSRL Beam Line 13-2. Oxygen-resonant X-ray emission and absorption spectroscopies provide the means to probe the density of states (DOS) projected on an atomic site in both occupied and unoccupied electronic states. Because of element specificity, the DOS of chemisorbed oxygen atoms can be disentangled from the substrate states and any change in the Pt-oxygen chemical bond can be probed directly.
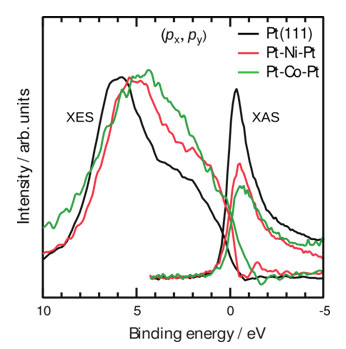
Figure 2 compares bonding and antibonding electronic states of oxygen chemisorbed on Pt-Ni-Pt and Pt-Co-Pt with ligand-free Pt-Pt-Pt surfaces. The occupied bonding states of O/Pt-Pt-Pt are characterized by a peak in the X-ray emission spectroscopy (XES) results at 6 eV and a broad shoulder reaching the Fermi level. Partially filled unoccupied antibonding states are seen as a strong resonance at 1 eV above the Fermi level in X-ray absorption spectroscopy (XAS) results. States between these will have nonbonding and antibonding characteristics. Subsurface Ni or Co layers change the nature of these states.
Two immediate effects are observed for all Pt-3d-Pt(111) surfaces: First, the intensity of the antibonding resonance in XAS lowers and the intensity of the antibonding shoulder (region between the Fermi level and 5 eV) in XES increases. The results clearly show the shift of the Pt-O antibonding states from above to below the Fermi level. The lowering and consequent increased population of the antibonding states is a direct consequence of the shift of the Pt 5d states to higher binding energies. The effect becomes larger, i.e., the XAS resonance continues to decrease and the XES antibonding shoulder continues to increase, when the subsurface 3d metal is changed from Ni to Co.
The degree of filling antibonding states is directly related to the strength of the Pt-O chemical bond and more filled antibonding states indicate a weaker Pt-O chemical bond. Thus, it enhances the reactivity of oxygen atoms since they are not strongly bound to the Pt surfaces.
This study shows that a simple ligand effect can play a big role in the reactivity of the surfaces of catalysts.
This work is supported by the Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under contract DE-AC02-76SF00515. This research was carried out at the Stanford Synchrotron Radiation Lightsource, a national user facility operated by Stanford University on behalf of the U.S. Department of Energy, Office of Basic Energy Sciences.
- T. Anniyev, S. Kaya, S. Rajasekaran, H. Ogasawara, D. Nordlund and A. Nilsson, "Tuning the Metal-Adsorbate Chemical Bond through the Ligand Effect on Platinum Subsurface Alloys", Angew. Chem., Int. Ed. Engl. 51, 7724 (2012) doi: 10.1002/anie.201201068




