Botulinum neurotoxins (BoNTs) are produced by Clostridium botulinum and cause the neuroparalytic syndrome of botulism. With a lethal dose of 1 ng/kg, they pose a biological hazard to humans and a serious potential bio-weapon threat (1). On the other hand, BoNTs have become a powerful therapeutic tool in the treatment of a variety of neurological, ophthalmic, and other disorders manifested by abnormal, excessive, or inappropriate muscle contractions. Experimental studies are also underway that explore the use of BoNTs in the management of chronic pain, such as headache and migraine. BoNTs bind with high specificity at neuromuscular junctions and they impair exocytosis of synaptic vesicles containing acetylcholine through specific proteolysis of SNAREs which constitute part of the synaptic vesicle fusion machinery (2,3). The molecular details of the toxin-cell recognition have been elusive.
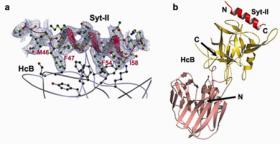
Using the X-ray diffraction data collected on SSRL beam line 9-1 and ALS beam line 8.2.2, Axel Brunger's group at Stanford University has determined the first crystal structure of a BoNT in complex with its protein receptor: the receptor binding domain (HcB) of botulinum neurotoxin serotype B (BoNT/B) bound to the luminal domain of synaptotagmin II (Syt-II), at 2.15 Å resolution (Figure 1). Upon binding a helix is induced in the luminal domain which binds to a saddle-shaped crevice on a distal tip of BoNT/B. This crevice is adjacent to the non-overlapping ganglioside binding site of BoNT/B (4,5) (Figure 2). Synaptotagmin II interacts with BoNT/B with nanomolar affinity, at both neutral and acidic endosomal pH. Biochemical and neuronal ex vivo studies of structure-based mutations indicate high specificity and affinity of the interaction, and high selectivity of BoNT/B towards the isoform II of synaptotagmin compared to isoform I. Synergistic binding of both synaptotagmin and ganglioside imposes geometric restrictions on the initiation of BoNT/B translocation upon endocytosis (Figure 2). These results could provide the basis for the rational development of preventive vaccines or inhibitors against these neurotoxins. Furthermore, identification of both receptor sites provides a new approach to retarget BoNTs to different cell types by site directed mutagenesis. Such modified BoNTs could also be used as drug delivery systems.
- Arnon, S. S. et al. (2001) Botulinum toxin as a biological weapon: medical and public health management. JAMA, 285, 1059-70.
- Schiavo, G. et al. (1992) Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature, 359, 832-5.
- Chen, Y. A., Scales, S. J., Patel, S. M., Doung, Y. C. & Scheller, R. H. (1999) SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell, 97, 165-74.
- Swaminathan, S. & Eswaramoorthy, S. (2000) Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat. Struct. Biol., 7, 693-9.
- Rummel, A., Mahrhold, S., Bigalke, H. & Binz, T. (2004) The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol. Microbiol., 51, 631-43.
Jin, R., Rummel, A., Binz, T., and Brunger, A. T. (2006) Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature, 444, 1092-1095.




