Tumour necrosis factor is a polypeptide cytokine involved in inflammation and the acute phase response. TNF-alpha is present in larger quantities in persons with rheumatoid arthritis or Crohn's disease. Direct inhibition of TNF-α by the commercial biological agents etanercept (Enbrel), infliximab (Remicade), adalimumab (Humira), has produced significant advances in rheumatoid arthritis treatment and validated the extra-cellular inhibition of this proinflammatory cytokine as an effective therapy. However, despite considerable incentives, viable leads for analogous small-molecule inhibitors of TNF-α have not been reported (1). Such drugs with attendant advantages in manufacturing, patient accessibility, administration, and compliance would represent a major advance in the treatment of TNF-α mediated diseases.
A group of Scientists at Sunesis Pharmaceuticals described a small molecule TNF-α inhibitor initially identified from an in vitro screen for compounds capable of disrupting TNF binding to TNF receptor R1. By using the x-ray diffraction data collected at Beam Line 7-1 at SSRL, He M.M. et al were able to determine a co-crystal structure of TNF-α with the small molecule inhibitor. The structure revealed a novel interaction in which one of the subunits of the TNF-α trimer is replaced by the small molecule (Fig. 1A). The resulting TNF-α dimer retained the same basic structural subunit fold as in the native trimer but the angle between the subunits within the dimer was slightly widened. The compound binds in the hydrophobic core of the protein that was completely buried within the subunit interfaces in the intact TNF-α trimer crystal structure. Interestingly, a large fraction of the contact surface with the small molecule involves six tyrosine residues from the TNF-α dimer (Fig. 1B). Further biophysical experiments conducted at biologically relevant TNF-α concentrations confirmed that the compound was capable of dissociating TNF-α trimer in solution and that the compound interacts with intact TNF-α trimer to dramatically accelerate subunit dissociation.
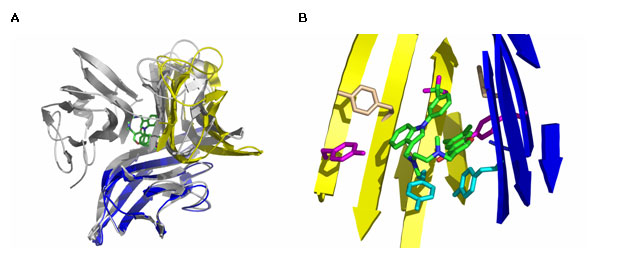
The results we have described should enable the design of appropriate assays that may allow for the identification of potent small-molecule inhibitors that inactivate multimeric proteins via a rapid predissociation-independent subunit dissociation process.
M. A. Palladino, F. R. Bahjat, E. A. Theodorakis, L. L. Moldawer, Nat. Rev. Drug Discov. 2, 736 (2003).
He, M.M., Smith, A.S., Oslob, J.D., Flanagan, W.M., Braisted, A.C., Whitty, A., Cancilla, M.T., Wang, J., Lugovskoy, A.A., Yoburn, J.C., Fung, A.D., Farrington, G., Eldredge, J.K., Day, E.S., Cruz, L.A., Cachero, T.G., Miller, S.K., Friedman, J.E., Choong, I.C., Cunningham, B.C. Small-molecule inhibition of TNF-alpha. Science v310 pp.1022-1025, 2005

