Over 4000 natural products contain halide atoms such as chlorine, bromine, or iodine.1 Halogenated natural products are medically valuable and include antibiotics (chlorotetracycline and vancomycin), antitumor agents (rebeccamycin and calichemycin), and human thyroid hormone (thyroxine).2 Halogenation is essential to the biological activity and chemical reactivity of such compounds, and often generates versatile molecular building blocks for synthetic organic chemists. Scientists have wondered about the mechanism by which proteins halogenate natural products, since the analogous reaction by organic synthesis poses multiple challenges. In many cases, the moiety to be halogenated is completely nonreactive, requiring employment of a powerful catalyst.
Researchers at the Massachusetts Institute of Technology used the macromolecular crystallography facilities at Stanford Synchrotron Radiation Laboratory to solve the first crystal structure of an iron-dependent halogenase (Fig 1A). This enzyme, SyrB2, from the plant pathogen Pseudomonas syringae catalyses the chlorination of threonine during biosynthesis of the anti-fungal agent syringomycin E.3 Unlike typical non-heme Fe(II)/α-ketoglutarate dependent enzymes that catalyze hydroxylation reactions, SyrB2 is a member of a new subclass of mononuclear iron enzymes that catalyze halogenation.
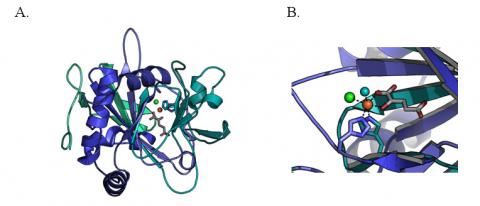
The structural analysis revealed a novel coordination environment for the catalytic iron and the presence of a naturally occurring iron-chloride bond. Blasiak et al used x-ray diffraction data collected at SSRL beamline 9-2 to pinpoint the location of the iron and halogen (Fig 1B). The carboxylate ligand that usually occupies one of the triangular sides of the octahedral iron coordination geometry in the hydroxylation reaction is replaced by a chloride iron. The active site architecture suggests a mechanism by which nature can harness the catalytic prowess necessary to perform the most chemically challenging of halogenation reactions. The typical mechanism of hydroxylation by non-heme iron enzymes involves formation of a high-valent oxoiron species that abstracts a hydrogen from the substrate.4 The resulting substrate radical then recombines with OH to give the alcohol product. The position of the chloride ligand in the SyrB2 active site presumably allows for rerouting of the substrate radical for recombination with chlorine radical, yielding the observed chlorinated product. This work provides an important step forward in understanding the biological synthesis of medically useful halogenated natural products.
This work was supported by NIH Grants GM 69857 (CLD), GM 49338 (CTW), a T32-GM08334 Training Grant (LCB), a Merck-sponsored Fellowship of the Helen Hay Whitney Foundation (FHV) and a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship (FHV).
- Gribble, G.W. Natural Organohalogens: A New Frontier for Medicinal Agents? J Chem Educ v81 pp.1441-1449, 2004
- Vaillancourt, F. H.; Yeh, E.; Vosburg, D. A.; Garneau-Tsodikova, S.; Walsh, C. T. Nature's Inventory of Halogenation Catalysts: Oxidative Strategies Predominate Chem. Rev. ASAP Article, 2006.
- Vaillancourt, F.H., Yin, J., Walsh, C.T. SyrB2 in syringomycin E biosynthesis is a nonheme FeII-ketoglutarate- and O2-dependent halogenase. Proc Natl Acad Sci USA v102 pp.10111-10116, 2005.
- Hausinger, R.P. Fe(II)/a-ketoglutarate-dependent hydroxylases and related enzymes Crit Rev Biochem Mol Biol v39 pp.21-68, 2004.
Blasiak, L.C., Vaillancourt, F.H., Walsh, C.T., Drennan, C.L. Crystal structure of the non-haem iron halogenase SyrB2 in syringomycin biosynthesis. Nature v440 pp.368-371, 2006




