Seasonal influenza A is a scourge of the young and old, killing more than 250,000 worldwide each year, while creating an economic burden for millions. Pandemic influenza, which occurs when a new virus emerges and infects people globally that have little or no immunity, represents a grave threat to human health. The recent spread of highly pathogenic avian influenza (HPAI) caused by the H5N1 strain across Asia, Europe and Africa raises the specter of a new pandemic, should the virus mutate to become readily transmissible from person to person. Influenza A is subclassified by its two major surface proteins: hemagglutinin, which mediates cell entry, first by recognizing host proteins bearing sialic acid on their surface, and second by triggering the fusion of viral and host membranes following endocytosis, allowing viral RNA to enter the cytoplasm; and neuraminidase, which cleaves sialic acid from host and viral proteins, facilitating cell exit. Hemagglutinin (HA or H), the major antigen on the flu virus surface, has a large globular head and a thin stem. The head of HA is constantly mutating, thereby helping the virus evade the immune system. As a result, the vaccine in the flu shot must be updated every year (Fig 1).
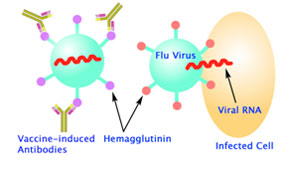
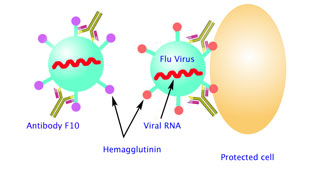
Fig 1B. (right) Antibody F10 target the stem of the HA, which rarely mutate and is common among many different subtypes of flu viruses. F10-bound HA can still bind to a cell, but it can no longer fuse the viral membrane with human cell membrane and inject RNA. Mice tested with the antibodies were protected from H5N1 flu in 80 percent of cases. (Image: William C. Hwang)
Sui Jianhua and colleagues led by Dr. Wayne Marasco at the Harvard Medical School and the Dana-Farber Cancer Institute were initially seeking ways to fight bird flu. She identified 10 antibodies, from a library of 27 billion human antibodies, against influenza hemagglutinin H5. The antibodies she isolated can neutralize bird flu in cell cultures when injected into mice (experiments conducted by Sandra Perez and other scientists led by Dr. Ruben Donis at the CDC). Remarkably, subsequent studies showed the same antibodies were also effective against all group 1 influenza viruses tested, including H5N1 'bird flu' and the 1918 H1N1 'Spanish flu' which killed millions around the world during World War I. Attempts to create mutant forms of the virus that could evade the antibody were unsuccessful.
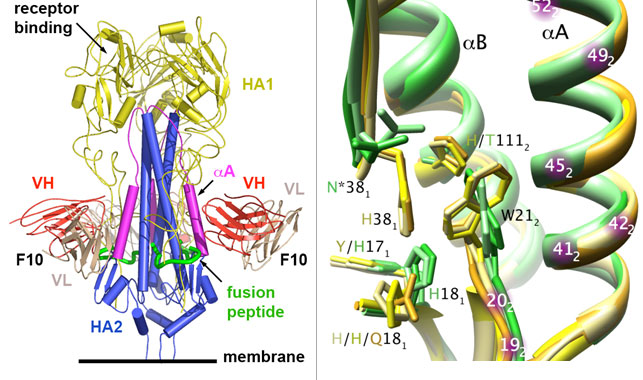
Fig 2B. (right) F10 epitope on HA can be classified by structure into two classes, corresponding the two phylogenetics groups (group1 and group 2) of HA. H1, H5, and H9 (group 1) are in shades of yellow (PDB 1RU7, 2IBX, and 1JSD); H3 and H7 (group 2) are in shades of green (PDB 1MQL and 1TI8). (Image: William C. Hwang; Source: Nature Structural & Molecular Biology)
Using data collected at SSRL, William Hwang and a group of colleagues led by Dr. Robert Liddington at the Burnham Institute for Medical Research determined the crystal structure of the hemagglutinin-antibody (H5-F10) complex to understand why the antibody could neutralize so many different types of influenza viruses (Fig 2). They found this antibody targets a highly conserved pocket, which mediates the fusion of viral membrane with host cells, in the stem region of hemagglutinin. This pocket seems to be critical for influenza's survival, and so it rarely changes its form, and is similar across many types of influenza. This explains why this antibody is so effective against so many different types of flu viruses. In addition, they found the epitope on the HA can be classified by structure into two classes corresponding to the two phylogenetics groups (group1 and group 2) of HA. F10 epitope belongs to class 1 (group 1). Experiments have shown that F10 can indeed neutralize all group 1 influenza viruses tested, but not group 2 viruses as expected. Flu viruses may eventually find a way to dodge this antibody. Nevertheless, this highly conserved epitope are less prone to mutation, and we can expect fewer escape mutants should they appear. The problem to defeat the flu virus will be much more tractable.
Single-handed flu combat? by Tia Ghose, The Scientist.
http://www.the-scientist.com/blog/display/55443/
Okuno, Y., Isegawa, Y., Sasao, F. & Ueda, S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 67, 2552-2558 (1993).
Smirnov YA, Lipatov AS, Gitelman AK, Okuno Y, Van Beek R, Osterhaus AD, Claas EC. An epitope shared by the hemagglutinins of H1, H2, H5, and H6 subtypes of influenza A virus. Acta Virol. 43, 237-244 (1999).
Varecková E, Mucha V, Wharton SA, Kostolansky F. Inhibition of fusion activity of influenza A haemagglutinin mediated by HA2-specific monoclonal antibodies. Arch Virol. 2003 Mar;148(3):469-86.
Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 16, 265 - 273 (2009).




