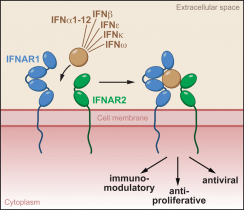
Type I interferons (IFNs) are secreted signaling proteins that belong to the family of cytokines. They are important mediators of the communication between cells and for innate immunity against viruses and cancer. Because of their pronounced antiviral, antiproliferative, and immunomodulatory properties, type I IFNs are used for the treatment of a broad range of human diseases, including hepatitis, multiple sclerosis, and several types of cancer 1. Type I IFNs act on, and are produced by almost every nucleated cell, and comprise 16 members in humans: IFNb, IFNe, IFNk, IFNw, and 12 subtypes of IFNa2. Although similar in their spectrum of activities, members of the type I IFN family differ in their signaling properties qualitatively and quantitatively; yet they all exert their physiological effects by binding to the same cell surface receptor consisting of two receptor chains called IFNAR1 and IFNAR2 (Fig. 1). The basis for the differential activity and signaling through a shared receptor has remained enigmatic. Using a combination of X-ray crystallographic, biochemical, and cell biology experiments, we were able to elucidate the mechanisms of ligand recognition and discrimination by the type I IFN receptor.
We determined the crystal structures of two human type I IFN receptor complexes containing different ligands, IFNw and a variant of IFNa, respectively (Fig. 2A and 2B). These two IFNs exhibit distinct biological activities. The structures revealed that the recognition mode and architecture of the complexes are unique among cytokine receptors, but conserved between different type I IFNs (Fig. 2C). Receptor-ligand cross-reactivity is based on “anchor point” residues shared among different IFNs. These anchor point residues are interspersed in the receptor-ligand interfaces amongst ligand-specific contacts that modulate the relative IFN binding affinities. Based on the ternary complex structures, a range of IFNa and IFNw mutants were designed that carried substitutions in contact residues.
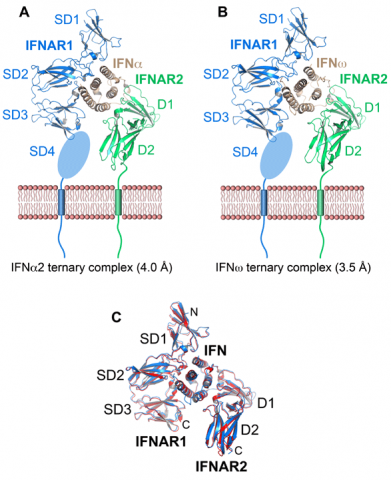
These mutants were characterized by affinity measurements, in antiproliferative and antiviral assays, with respect to their potency to induce gene expression, in a receptor downregulation assay, and by cell signaling studies using cells of whole blood from human donors. The experiments with the IFN interface mutants allowed us to conclude that ligand discrimination by the receptor occurs primarily through different energetics of the shared receptor contacts and, to a lesser extent, through the IFN subtype-specific contacts. The resulting specific ligand recognition chemistries lead to different receptor-ligand complex stabilities, which, in conjunction with a ligand-induced conformational change in IFNAR1, control signal initiation, resulting in functional differences between IFNs. Mechanistically, different complex stabilities result in different receptor internalization rates and might control the relative Janus kinase activities toward cytoplasmic substrates, leading to distinct levels of downstream effector activation that ultimately manifest in distinct gene expression patterns. By forming a gradient of complex stabilities, IFNs induce specific cellular signaling profiles that form the basis for the diverse biological activities observed for these cytokines.
Using the newly gained structural and functional knowledge of the IFN receptor system, we were able to rationally design a single-substitution IFNw interface mutant with higher affinity for IFNAR2 and significantly increased antiproliferative activity. This corroborates the model that IFN-specific polymorphisms in the interfaces influencing complex stability play a major role in determining IFN-specific functional activities. Thus, our studies not only provide fundamental insights into one of the most important systems of innate immunity, but also enable us to tune the activity of IFN ligands and design cytokines with customized signaling properties for research and therapeutic purposes.
K.C.G. is an Investigator of the Howard Hughes Medical Institute. This work was also supported by NIH-R01-AI51321 (K.C.G.) and NIH-R01-AI087917 (J.S.G.); C.T. was supported by a long-term postdoctoral fellowship of the International Human Frontier Science Program Organization. G.S. and J.P. are supported by the European Community's FP7/2007-2013 under GA no. 223608 (IFNaction). X-ray crystallographic data sets used in this study were collected at SSRL beamlines 9.1, 9.2, and 11.1, and at beamlines 8.2.1 and 8.2.2 of the Advanced Light Source (ALS), Berkeley.
1. Borden, E.C. et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov 6, 975-90 (2007).
2. Pestka, S., Krause, C.D. & Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol Rev 202, 8-32 (2004).
Thomas, C., Moraga, I., Levin, D., Krutzik, P.O., Podoplelova, Y., Trejo, A., Lee, C., Yarden, G., Vleck, S.E., Glenn, J.S., Nolan, G.P., Piehler, J., Schreiber, G., Garcia, K.C. Structural linkage between ligand discrimination and receptor activation by type I interferons. Cell 146, 621-32 (2011).




