Most eukaryotic viruses, including HIV, influenza and herpes viruses, undergo maturation when transitioning from the noninfectious provirion to the infectious virion. Maturation processes involve reorganization of viral quaternary structure to defend viral gene from the cellular defense mechanism and lead to effective transfection. Nudaurelia capensis omega virus, NwV, is a T=4, non-enveloped, icosahedral, single strand RNA virus, where T is the triangulation number defining an icosahedral lattice of the virus capsid structure. Virus like particles (VLPs) of NwV exhibit large pH-dependent conformational changes (LCC) when the procapsid, purified at pH=7.6, (~480 Å) is exposed to pH=5.0, resulting in ~400 Å particles (Figure 1). In response to the LCC, an auto-proteolysis occurs in which each of 240 subunits is cleaved at Asn570-Phe571 (1). We investigated this pH-induced maturation by equilibrium and time-resolved small angle X-ray scattering (SAXS) at SSRL beam line 4-2.
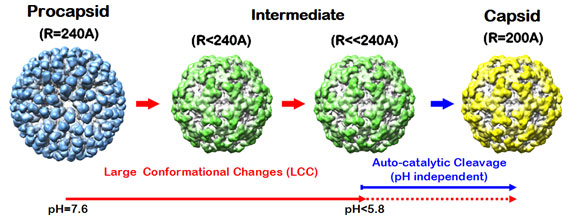
We showed that when the acidic interfaces of the NwV subunits are protonated the electrostatic repulsion between adjacent subunits is weakened, allowing the particle to undergo spontaneous size reduction through a LCC. This condensation was studied at different pH values allowing the determination of a titration curve that demonstrated a continuous change in particle size with an overall particle pKa = ~5.8. An N570T mutation of the NwV subunits, that does not undergo the maturation cleavage, shows identical behavior to the wild type at pH values between 7.6 and 6.0. However, they exhibit different maturation properties at pH values between 5.8 and 5.0, with the mutant (uncleaved) particle displaying systematically larger radii above pH 5.0. To directly determine the relationship between cleavage and particle size, time resolved SAXS studies were carried out at pH 5.5 (the maximum pH at which 100% cleavage occurs in 3 days) (Figure 2). At pH=5.5, auto-proteolysis was required to achieve the final mature size, whereas particles below pH 5.0 did not require cleavage to achieve the final mature size.
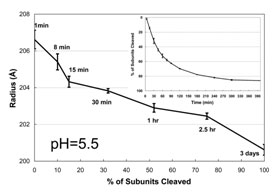
The particle radius decreases from 236 Å to 207 Å in one minute and then slowly decreases in size to ~200 Å as shown. The size change is closely proportional to the fraction of subunits cleaved, emphasizing the role of cleavage at this pH in the final stages of particle condensation. The kinetics of particle cleavage at pH 5.5 is shown in the Figure 2 inset (1). The results indicated that structural forces prior to cleavage counter the reduction in electrostatic force and that the structural resistance due to the repulsive intersubunit interaction is reduced when the auto-proteolysis occurs. These studies also show that there is a significant period of protein annealing required for the capsid to reach its equilibrium dimension. Based on the crystallographic structure of the fully mature virion, we hypothesize that the annealing involves positioning of the molecular switches associated with the T=4 quasi-symmetry as well as the formation of the active site for auto-proteolysis.
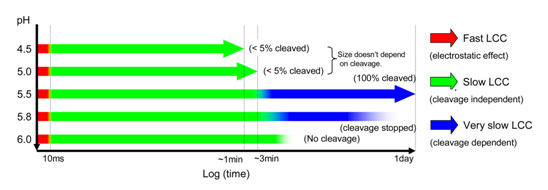
Finally, fast time resolved studies were performed at different pH values (Figure 3). The time resolution for the experiment is ~10 ms, and data frames were recorded successively after the drop in pH using a stopped-flow mixing apparatus. The results demonstrated 3 kinetic stages in the particle condensation with each incremental drop in pH, the first completing within 10 ms, the second in less than 5 seconds and the third in the 2-3 minute time regime corresponding to the annealing described above. In addition to those stages, the slow cleavage dependent stage (hours) is required at pH values between 5.8 and 5.5 (Figure 2). Those maturation events at the quaternary structure level are reminiscent of protein folding, where there is rapid formation of a molten globule, followed by different stages of polypeptide and side-chain annealing.
The study breaks new ground in understanding the energy landscape associated with virus maturation, a process common in complex human viruses, and required for a provirion to become an infectious virion. Encoded within the provirion structure is a program that strengthens the capsid and activates an auto-catalytic cleavage of the subunits.
1. Matsui, T., Lander, G. and Johnson, J.E. Characterization of Large Conformational Changes and Auto-proteolysis in the Maturation of a T=4 Virus Capsid. J. Virol., 83, 1126-1134 (2009).
Matsui, T., Tsuruta, H. & Johnson, J.E. Balanced Electrostatic and Structural Forces Guide the Large Conformational Change Associated with Maturation of T=4 Virus. Biophys. J., 98, 1337-1343 (2010).




