The emergence of drug-resistant microbes represents a major impediment in the treatment of bacterial infections. Resistance to first-choice drugs has become problematic for respiratory infections, AIDS, tuberculosis, malaria and diarrheal diseases, which are top killers among infectious agents. When second- and third-choice drugs succumb to similar resistance, treatment options become dire. As such, a major scientific priority in health-related research and medicine is to identify new antimicrobial targets and to develop novel drugs that keep infectious diseases in check according to global demand.
Riboswitches are a novel class of molecules that regulate the production of as many as 4% of bacterial genes. In the classical view of gene expression, instructions stored in DNA are copied into mRNAs, which make their way to the ribosome where the genetic information is decoded as a protein. Groundbreaking work by the Breaker lab at Yale and Nudler lab at NYU demonstrated in 2002 that mRNA serves as more than a passive carrier of genetic information in this decoding process. On the contrary, each group showed that key regions of certain mRNAs are capable of "sensing" specific cellular metabolites. This process occurs when conserved regions within the mRNA adopt a three-dimensional fold within a so called 'aptamer domain' that embraces the smaller metabolite molecule with high affinity - often at the nM level. The significance of this recognition is that the 'folded' state of the mRNA sequesters critical signals needed for successful protein production by the processes of transcription or translation. Genetic regulation is achieved because the metabolite is often produced by a protein whose mRNA harbors the riboswitch sequence, thus providing direct feedback. When there is too much metabolite, the riboswitch clenches its metabolite partner to inactivate expression. As levels of the metabolite drop, the riboswitch relaxes its grip, exposing signals that favor mRNA expression. Understanding how to manipulate a given riboswitch's "on" and "off" states offers the prospect of a novel antimicrobial target, especially when the associated genes encode metabolic intermediates that influence the pathogenicity or survival of the bacterium.
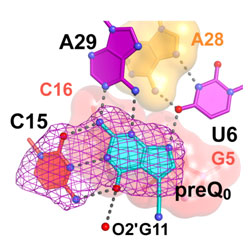
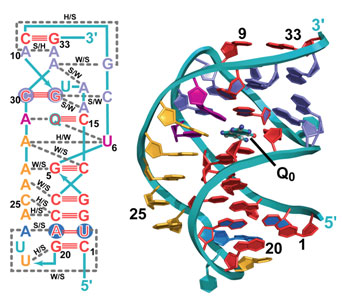
The mode of gene regulation by riboswitches represents a new paradigm for the field but poses key questions: What is the minimal size of an aptamer? Is the mode of metabolite recognition and expression-signal sequestration universal for a given riboswitch class? In the current study, Joseph Wedekind's lab at the University of Rochester (NY) solved the crystal structure of the smallest, naturally occurring riboswitch aptamer known as preQ1 (Fig. 1), which was derived from a hot spring bacterium Thermoanaerobacter tengcongensis, and is bound to the guanine-like metabolite preQ0 (Q0). Wedekind's group utilized beam line 7-1 at SSRL, which was upgraded recently to allow multi-wavelength anomalous diffraction and MAD phasing experiments. Dr. Wedekind and his team accessed 7-1 remotely from Rochester, and have the distinction of solving the first MAD structure on the improved line. The crystal structure revealed a highly compact RNA fold in which more than 50% of all nucleotides are engaged in base triplet interactions (Fig. 1). When the riboswitch twists into the well-known double helix structure, the RNA bases align in the chain such that they stack on top of each other, adding stability. Because Q0 is a modified form of the guanine base, its presence in a solvent-sequestered riboswitch binding pocket bridges a gap between otherwise unstacked RNA bases (Fig. 1), thus contributing to the ~100 nM affinity of the aptamer domain for the metabolite. Specificity for Q0 is achieved by "read out" of the metabolite by C15 of the aptamer, which is reminiscent of the mode by which a G:C pair forms in DNA (Fig. 2); other conserved bases assist in Q0 recognition, such as U6 and A29. Perhaps most importantly, the new structure revealed that the first base of the mRNA's ribosome binding site, G33, pairs with a C9 of the riboswitch loop, which ties up the critical signal necessary for mRNA decoding. Prior biochemical studies support the importance of maintaining the preQ1 metabolic pathway for efficient bacterial growth, and evidence suggests that the pathway is required for pathogenicity. Future investigations will focus on whether the mode of metabolite recognition is universal or species specific, which has implications for targeting various subgroups of bacteria and the development of new antimicrobials.
This work was support in part by grants from the NIH (GM63162) and the donors of the Petroleum Research Fund to JEW. RCS was supported in part by an Elon Huntington Hooker graduate fellowship.
Blount, K. F., and Breaker, R. R. (2006) Nat. Biotechnol. 24, 1558-1564.
Roth, A., Winkler, W. C., Regulski, E. E., Lee, B. W., Lim, J., Jona, I., Barrick, J. E., Ritwik, A., Kim, J. N., Welz, R., Iwata-Reuyl, D., and Breaker, R. R. (2007) Nat. Struct. Mol. Biol. 14, 308-317.
Iwata-Reuyl, D. (2003) Bioorg. Chem. 31, 24-43.
Editorial Note (2009) Minimal Recognition Required. J. Biol. Chem. 284, e99911.
Spitale R.C., Torelli A.T, Krucinska J., Bandarian V., Wedekind J.E. (2009) The structural basis for recognition of the PreQ0 metabolite by an unusually small riboswitch aptamer domain. J. Biol. Chem. 284, 11012-6.




