The development of rechargeable batteries has played an important role in advancing portable electronic devices and electric vehicles (EVs). The current generation batteries still require improvements on energy density for extended EV range and cell phone/laptop usage time on a single charge. To further increase the battery energy density, Li-alloying reactions with metal and intermetallic compounds (such as Si, Sn, and SnSb) are promising candidates because of their higher energy density compared to the current anode material graphite. However, the alloy-type anode cycling stability is typically poor. The battery capacity can decay up to 50% after 10 charge – discharge cycles, which is mainly due to the high volumetric changes during the battery cycling process. For example, Sn can expand by more than 300% after complete reaction with lithium, causing electrodes to fracture. This may also lead to other capacity degradation pathways, including continuous electrolyte decomposition and disconnected electrode fragments.
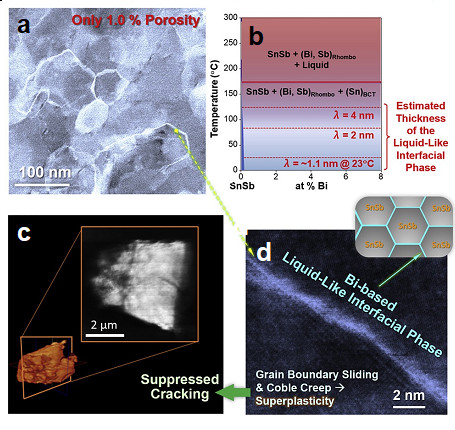
In a recent study, researchers led by University of California San Diego (UCSD) nanoengineering professor Jian Luo and Ph.D. student Qizhang Yan, in collaboration with scientists SSRL and University of California Los Angeles (UCLA), demonstrate a method to suppress alloy-type anode cracking via a thermodynamics-driven grain boundary engineering. More specifically, Bi addition in SnSb is used to substantially improve the SnSb cycling stability, achieved even in dense micrometer-sized secondary particles of nanocrystalline alloys with only 1% porosity. Previously, such performance can only be achieved in nanoporous materials. Cryogenic transmission electron microscopy and thermodynamic modeling were used to reveal the formation of Bi-enriched liquid-like interfacial phase at SnSb grain boundaries. The cracking suppression mechanism was studied with synchrotron-based full-field transmission x-ray microscopy (TXM) at SSRL BL6-2c via 3D tomography of cycled electrode particles and in situ 2D TXM imaging during battery cycling.
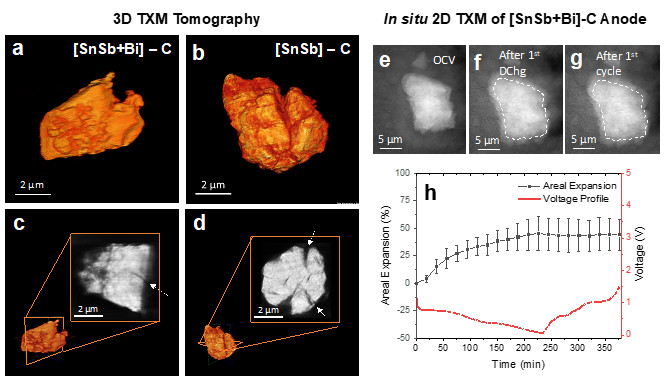
The results suggest that the liquid-like interfacial phase can form spontaneously at a thermodynamic equilibrium to serve as a stress relief mechanism for the high volumetric expansion anode via promoting grain boundary sliding and Coble creep, akin to the room-temperature superplasticity known for Sn-Bi alloys. This work suggests the thermodynamically driven interfacial engineering as a new approach to improve the stability of high-volume expansion battery electrode materials. It creates a new class of dense nanostructured volume-expansion anode materials that can be well cycled.
This work is supported as part of the Center for Synthetic Control Across Length-scales for Advancing Rechargeables (SCALAR), an Energy Frontier Research Center funded by the United States Department of Energy. Research teams led by Prof. Sarah H. Tolbert (UCLA), Dr. Johanna Nelson Weker (SSRL), and Prof. Bruce S. Dunn (UCLA) from SCALAR EFRC contributed to this work.
Q. Yan, S.-T. Ko, A. Dawson, D. Agyeman-Budu, G. Whang, Y. Zhao, M. Qin, B. S. Dunn, J. Nelson Weker, S. H. Tolbert and J. Luo, "Thermodynamics-driven Interfacial Engineering of Alloy-type Anode Materials”, Cell Rep. Phys. Sci. 3, 100694 (2022) doi: https://doi.org/10.1016/j.xcrp.2021.100694

