The ability of organisms to sense and respond to light availability is of great interest to those wishing to exploit these mechanisms in the areas of biotechnology and agriculture. A particular light sensing domain, termed the light-oxygen-voltage (LOV) domain, is found in plants, animals, and bacteria to control a wide variety of biological processes1-5. LOV domains oftentimes signal to a variety of attached effector domains including kinases, F-boxes, and DNA-binding domains. This leads to the questions: How does light stimulation of the LOV domain cause activation to such a wide variety of effector domains? And is there a shared mechanism? Understanding the molecular mechanism of how organisms use LOV domains might enable engineered light-induced control of pathways.
El222 is a two-domain 24-kDa protein consisting of a light-sensing LOV domain and a LuxR-like DNA binding domain. The LOV domain has the traditional PAS fold of b2a4b3 surrouding a central flavin (FMN) chromophore. Upon blue-light stimulation at 450 nm, electrons in the FMN isoalloxazine ring delocalize and a covalent bond is formed between the FMN C4a carbon and the conserved Cys75 in the El222 LOV domain.
Diffraction data were collected at SSRL Beam Line 9-1. Since structures of isolated LOV and HTH domains from other organisms were known, we used molecular replacement to solve the structure of the first naturally occurring sensor/effector pair. The Ja linker helix was not used in the initial search but subsequently built by hand into positive difference density that illustrated a connection between the two domains. The structure was iteratively refined to a resolution of 2.1 Å.
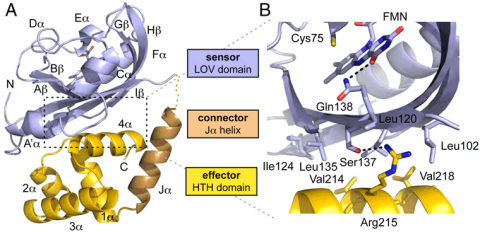
LuxR-like HTH domains dimerize via their 4a helices and subsequently bind to the major groove of DNA via their DNA recognition helix 3a. In El222, the HTH domain is unable to dimerize in the dark state because it interacts with the LOV domain b-sheet (Fig. 1A). The interface between the two domains is quite hydrophobic with one hydrogen bond between Ser137 and Arg215 (Fig. 1B). In elucidating how light stimulation of the LOV domain can traverse the LOV domain to affect the HTH domain, we found a hydrogen bond network connecting the LOV domain to the HTH domain. In the dark state, the FMN carbonyl is hydrogen bonding with Gln138. The adjacent residue, Ser137, hydrogen bonds to Arg215 on the HTH domain. Thus it is plausible that the light-induced Cys75-FMN adduct propagates steric and electronic changes through the b-sheet to modulate LOV domain interaction with the HTH domain.
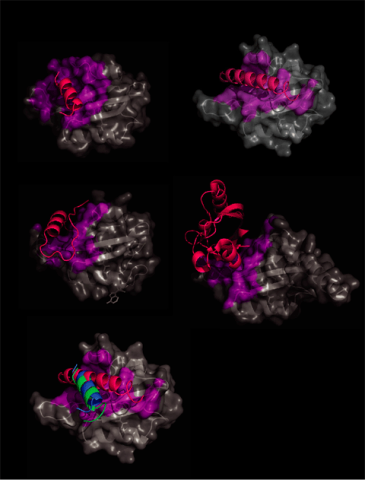
In order to confirm the FMN b sheet signaling mechanism for other LOV domains, we analyzed currently deposited LOV domain-containing structures for interactions with the LOV core. All external LOV domain interactions of at least 5 Å were highlighted on the LOV core (Fig. 2). In all cases external portions interact with the LOV domain b sheet in a similar fashion.
Furthermore, based on our crystal structure, we proposed a site-directed single amino acid mutation at the b sheet interface that we predicted would disrupt the interaction between the LOV domain b sheet and the HTH domain 4a helix. Unlike the wild type, which requires light for DNA binding, this engineered construct shows DNA binding even in the dark and is this constitutively active.
This work has implications for having a light-controlled expression system. Using light-controlled proteins to modulate cell activity is part of an emerging field called optogenetics. In addition to the obvious research uses, the concept is that light-based therapy might one day supplement or completely replace pills.
1. B.L. Taylor & I.B. Zhulin PAS Domains: Internal Sensors of Oxygen, Redox Potential, and LightMicrobiol Mol Biol Rev 63, 479-506 (1999).
2. E. Huala et al. Arabidopsis NPH1: A Protein Kinase with a Putative Redox-Sensing DomainScience, 278, 2120-2123 (1997).
3. E. Liscum & W.R. Briggs Mutations in the NPH1 Locus of Arabidopsis Disrupt the Perception of Phototropic Stimuli. Plant Cell, 7, 473-485 (1995).
4. T. Imaizumi et al. FKF1 is Essential for Photoperiodic-Specific Light Signalling in Arabidopsis Nature, 426, 302-306 (2003).
5. M. Avila-Perez et al. Blue Light Activates the SigmaB-Dependent Stress Response of Bacillus Subtilis via YtvA. J. Bacteriol., 188, 6411-6414 (2006).
A.I. Nash, R. McNulty, M.E. Shillito, T.E. Swartz, R.A. Bogomolni, H. Luecke, K.H. Gardner Structural Basis of Photosensitivity in a Bacterial Light-Oxygen-Voltage/Helix-Turn-Helix (LOV-HTH) DNA-Binding Protein PNAS, 108, 9449-9454 (2011).




