Acute lymphoblastic leukemia (ALL) is an aggressive lymphoid malignancy that is currently the leading cause of cancer in pediatric patients1. Despite intensified chemotherapy regimens, the cure rates of ALL only approaches 40%2. Specific mutations in the cytosolic 5’-nucleotidase II (NT5C2) gene are present in about 20% of relapsed pediatric T-ALL and 3-10% of relapsed B-precursor ALL cases3,4.
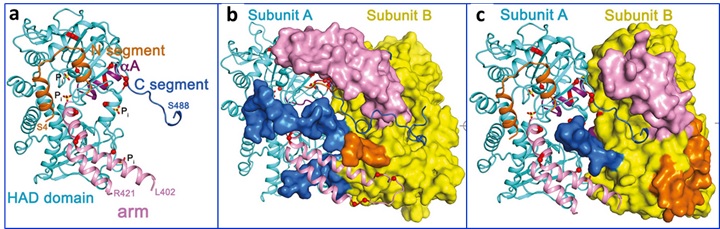
NT5C2 is a cytosolic nucleotidase that maintains intracellular nucleotide pool levels by exporting excess purine nucleotides out of the cell5. NT5C2 can also dephosphorylate and inactivate the metabolites of the 6-thioguanine (6-TG) and 6-mercaptopurine (6-MP) commonly used to treat ALL6. Thus, relapse associated activating mutations in NT5C2 confer resistance to 6-MP and 6-TG chemotherapy. Upon allosteric activation, a disordered region of NT5C2 adopts a helical configuration (helix A) and facilitates substrate binding and catalysis (Fig. 1a)7. Mutations in this regulatory region of NT5C2 have been modeled to strongly activate NT5C2. However, the majority of NT5C2 mutations associated with relapsed ALL do not occur in this region.
To better understand the mechanisms by which these gain-of function NT5C2 mutations lead to increased nucleotidase activity, Dieck, Tzoneva, Forouhar and colleagues investigated additional regulatory elements that may control NT5C2 activation. They collected crystallographic data for several mutant NT5C2 homotetramers at SSRL (NT5C2-537X D52N/D407A in active state (BL9-2), NT5C2-Q523X D52N in basal state and in active state (BL14-1) and full-length NT5C2 R39Q/D52N in basal state (BL12-2)) and used the structural information as a guide in the understanding of the mechanistic details.
The analysis of 15 structures along with structural modeling and genetic and functional antibody analyses revealed three classes of NT5C2 mutations with different mechanisms of action (Fig. 1). The Class I mutations lock the allosterically activated helix A in a constitutively active configuration. The Class II mutations, accounting for >95% of mutations, result in loss of an intramolecular NT5C2 switch-off mechanism responsible for returning NT5C2 to its basal inactive state following allosteric activation. The Class III mutations favor allosteric activation of NT5C2 due to the loss of a C-terminal acidic tail that functions as a brake towards NT5C2 activation. All together, these findings identify three activating mechanisms by which NT5C2 mutations increase nucleotidase activity and pave the way for the development of NT5C2 inhibitors to reverse thiopurine chemotherapy resistance in patients with relapsed ALL.
- D. A. Siegel et al., "Cancer Incidence Rates and Trends among Children and Adolescents in the United States, 2001-2009", Pediatrics 134, e945 (2014), doi:10.1542/peds.2013-3926
- S. P. Hunger and C. G. Mullighan, "Acute Lymphoblastic Leukemia in Children", New Engl. J. Med. 373, 1541 (2015) doi:10.1056/NEJMra1400972
- G. Tzoneva et al., "Activating Mutations in the NT5C2 Nucleotidase Gene Drive Chemotherapy Resistance in Relapsed ALL" Nat. Med. 19, 368 (2013) doi:10.1038/nm.3078
- J. A. Meyer et al., "Relapse-specific Mutations in NT5C2 in Childhood Acute Lymphoblastic Leukemia", Nat. Genet. 45, 290 (2013) doi:10.1038/ng.2558
- S. A. Hunsucker, B. S. Mitchell and J. Spychala, "The 5'-nucleotidases as Regulators of Nucleotide and Drug Metabolism", Pharmacol. Therapeut. 107, 1 (2005) doi:10.1016/j.pharmthera.2005.01.003
- C. Brouwer et al., "Role of 5'-nucleotidase in Thiopurine Metabolism: Enzyme Kinetic Profile and Association with thio-GMP Levels in Patients with Acute Lymphoblastic Leukemia during 6-mercaptopurine Treatment", Clinica Chim. Acta 361, 95 (2005) doi:10.1016/j.cccn.2005.05.006
- K. Wallden et al., "Crystal Structure of Human Cytosolic 5'-nucleotidase II: Insights into Allosteric Regulation and Substrate Recognition", J. Biol. Chem. 282, 17828 (2007) doi:10.1074/jbc.M700917200
C. L. Dieck, G. Tzoneva, F. Forouhar, Z. Carpenter, A. Ambesi-Impiombato, M. Sanchez-Martin, R. Kirschner-Schwabe, S. Lew, J. Seetharaman, L. Tong and A. A. Ferrando, "Structure and Mechanisms of NT5C2 Mutations Driving Thiopurine Resistance in Relapsed Lymphoblastic Leukemia", Cancer Cell 34, 136 (2018) doi: 10.1016/j.ccell.2018.06.003




