Arsenic (As) is a toxic metalloid which has attracted the attention of the general public because of its natural toxic concentrations in drinking water of millions of people around the world. The mobility and bioavailability of As thereby strongly depends on redox conditions, often linked to the redox cycles of sulfur (S), iron (Fe), and carbon (C). In reducing systems such as wetlands (swamps, peatlands, paddy fields etc.) As is thought to be mainly present in its reduced trivalent form as arsenite. Naturally, these systems are rich in natural organic matter (NOM) because mineralization of carbon is delayed under anoxic, reducing conditions. Furthermore sulfur, which acts as a main nutrient for plants, can also be present in its reduced forms as e.g. organic thiol groups in NOM-rich environments after anoxic decomposition of plant debris or reduction of released sulfate.
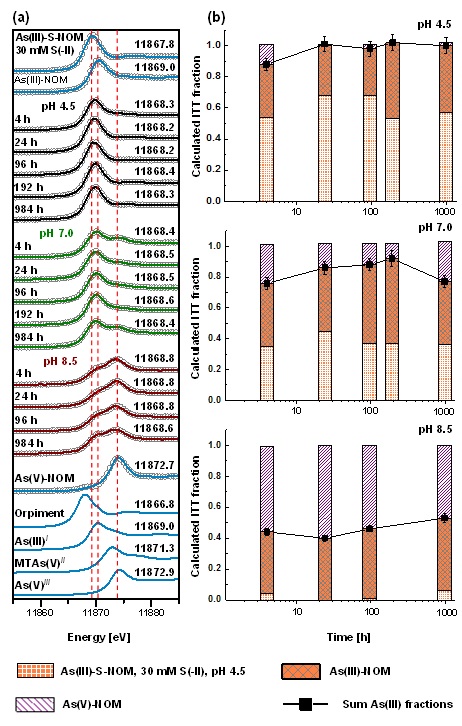
Figure 1: Example of time series from experiments where 50 µM monothioarsenate were added to sulfide-reacted peat at pH 4.5, 7.0 and 8.5. Panel (a) shows normalized XANES sample spectra and their reconstruction with three components after iterative target factor analysis (ITFA). Panel (b) shows the respective proportions of the three identified reference components after iterative target transformation (ITT). Reprinted with permission from Besold et al. 2018, ES&T, DOI: 10.1021/acs.est.8b01542, Copyright 2018, American Chemical Society.
Recent studies provided spectroscopic evidence that arsenite binding to NOM via organic thiol groups is an important mechanism for sequestering As in synthetic1 and natural systems.2 However, it is also known that under reducing conditions and in the presence of sulfide or zerovalent sulfur species, thio-arsenic species can form3, but yet have never been detected in NOM-rich environments.
Researchers from Bayreuth University in collaboration with colleagues from ETH Zürich and SLU Uppsala as well as Beam Line scientists from SSRL and ESRF have now studied thio-arsenic species occurrence and importance for As sequestration in the already broadly studied2 naturally As-enriched peatland Gola di Lago, Switzerland.
The research team investigated the aqueous As speciation in the porewater of five peat profiles (up to 2.1 m depth) with increasing distance from an arsenic-rich inflow by use of anion exchange chromatography coupled to inductively coupled mass spectroscopy (AEC-ICP-MS) and found that up to 93% of total aqueous As were actually thioarsenates. The main arsenic species monothioarsenate (MTAs(V), up to 91%) probably formed by a reaction of arsenite with surface associated zerovalent sulfur and showed highest concentrations in peat layers close to the surface. But with increasing depth, arsenite seemed to be present at the expense of MTAs(V).
To study this interesting behavior in more detail, anoxic batch experiments with peat high in organic thiol groups were conducted with 50 µM MTAs(V) at slightly acidic (pH 4.5), neutral (pH 7.0) and slightly alkaline (pH 8.5) conditions. The aqueous As speciation was monitored by AEC-ICP-MS and the solid-phase speciation by x-ray absorption spectroscopy over a period of 41 days.
In the aqueous phase, MTAs(V) quickly transformed to arsenite (half-life: ~35 h) at pH 4.5, whereas at pH 8.5 no transformation of MTAs(V) was observed. On the solid-phase, no thio-As species could be detected at all, but it was possible to follow increasing complexation of arsenite with mainly organic thiol groups of the peat. The complexation to thiol groups was highest and fastest at pH 4.5 and decreased to almost zero at pH 8.5 (Figure 1). These findings suggest that MTAs(V) is highly mobile at neutral to alkaline conditions, but at slightly acidic pH, MTAs(V) transformed quickly to arsenite in the aqueous phase and was subsequently effectively sequestered by organic thiol groups of the peat.
The results of the batch experiments fit mainly the observations at the field-site Gola di Lago and lead in combination with knowledge from the literature to the conceptual model of As-S chemistry shown in Figure 2. The processes suggested might also be representative for other As-enriched, sulfidic environments such as peatlands and swamps naturally enriched in As, as well as engineered wetlands used for removal of As and other metal(loid)s from mining effluents.
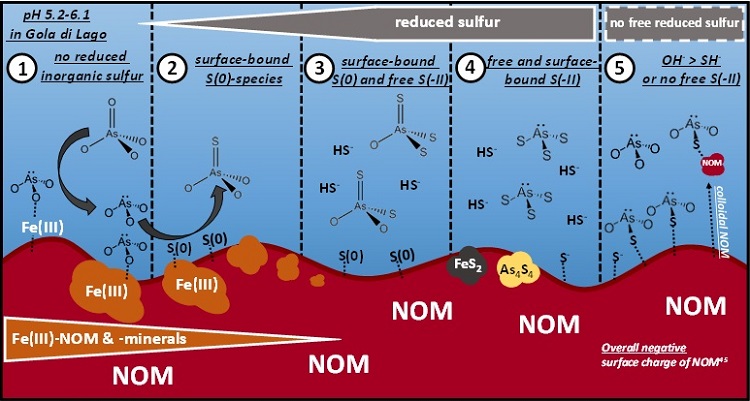
Figure 2: Proposed conceptual model for the As-S chemistry in the minerotrophic peatland Gola di Lago, Switzerland. Scenario 1: arsenate and arsenite prevail as long as no reduced inorganic sulfur is present. Scenario 2: monothioarsenate formation from arsenite and surface-bound zerovalent sulfur species. Scenario 3: formation of higher thiolated arsenates from monothioarsenate under conditions of available free sulfide. Scenario 4: thioarsenite formation and eventually FeS2 and As4S4 precipitation in sulfide-rich, strongly reducing micro-environments or during sulfide-rich times. Scenario 5: arsenite sorption to sulfhydryl groups of peat or colloidal NOM. Scenario 3 and 4 seem to be rather unlikely in Gola di Lago peatland since no free sulfide was detected. Reprinted with permission from Besold et al. 2018, ES&T, DOI: 10.1021/acs.est.8b01542, Copyright 2018, American Chemical Society.
- M. Hoffmann, C. Mikutta and R. Kretzschmar, "Bisulfide Reaction with Natural Organic Matter Enhances Arsenite Sorption: Insights from X-ray Absorption Spectroscopy", Environ. Sci. Technol. 46, 11788 (2012)
- P. Langner, C. Mikutta and R. Kretzschmar, "Arsenic Sequestration by Organic Sulphur in Peat", Nat. Geosci. 5, 66 (2012)
- B. Planer-Friedrich, C. Härtig, R. Lohmayer, E. Suess, S. H. McCann and R. Oremland, "Anaerobic Chemolithotrophic Growth of the Haloalkaliphilic Bacterium Strain MLMS-1 by Disproportionation of Monothioarsenate", Environ. Sci. Technol. 49, 6554 (2015)
J. Besold, A. Biswas, E. Suess, A. C. Scheinost, A. Rossberg, C. Mikutta, R. Kretzschmar, J. P. Gustafsson and B. Planer-Friedrich, "Monothioarsenate Transformation Kinetics Determining Arsenic Sequestration by Sulfhydryl Groups of Peat", Environ. Sci. Technol., in press (2018) DOI: 10.1021/acs.est.8b01542




