Anthrax Toxin is a major virulence factor in the infectious disease, Anthrax (1). This toxin is produced by Bacillus anthracis, which is an encapsulated, spore-forming, rod-shaped bacterium. Inhalation anthrax, the most deadly form, is contracted through breathing spores. Once spores germinate within cells of the immune system called macrophages (2), bacterial cells are released into the bloodstream. There they proliferate rapidly and secrete Anthrax Toxin, ultimately leading to septic shock and death. Although antibiotics may be used to kill the bacteria, the level of toxin has often become so high in the bloodstream that removing the bacteria alone is not sufficient to prevent death. Therefore, the design of anti-toxins offers the prospect of treatment in the advanced stages of infection. Together with collaborators from the NIH and Harvard Medical School, we are involved in the atomic resolution study of the Anthrax Toxin components and their complexes, including small molecules with therapeutic potential. Data collection at SSRL and other synchrotron radiation sources has been key to the advances made in this research so far and is expected to play a continuing role in the future.

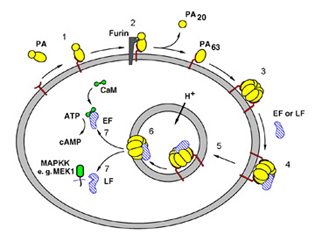
Anthrax Toxin, secreted into the bloodstream, is composed of 3 individual proteins - Protective Antigen (PA), Lethal Factor (LF) and Edema Factor (EF), which act in concert to disrupt cellular signaling systems in the host macrophage (Figure 1). PA binds a receptor on the host cell surface, is cleaved and activated by a host protease to form PA63, and then forms a seven-membered ring structure that binds the two toxic enzymes, LF and EF (1,3,4). The resulting toxin complex is engulfed (endocytosed) into intracellular compartments called endosomes. Natural cellular processes lead to acidification of the endosome, which triggers a conformational change in PA63, causing it to insert into the endosomal membrane and translocates the toxic enzymes into the host cell interior (cytosol). Once inside, LF and EF do their damage to the host cell defense system. LF is a metalloproteinase that cleaves six members of the MAPKK family (5) of intracellular signaling proteins, removing the specific fragment from individual MAPKKs that is crucial for immediate interaction with other signaling proteins. This action by LF rapidly blocks the signals that would normally recruit other immune cells to fight the infection (6). EF, on the other hand, is a calmodulin-activated adenylyl cyclase that increases the concentration of a messenger molecule (cAMP) needed for regulated cell functions (7) to abnormal levels, causing accumulation of fluids within and between cells, and hence edema. The disruption of normal signaling pathways result in cell lysis, the sudden release of messenger molecules, and toxic shock.
The three-dimensional atomic resolution structures of all three individual components (PA (4), LF (8), and EF (9)) have now been solved by X-ray crystallography. We recently reported the crystal structure of Lethal Factor and its complex with a 16-amino acid residues long (16-mer) peptide representing the N-terminus of its natural substrate, MAPKK-2, in Nature (23 October online pre- release and 8 November 2001 in print) (8). Two different crystal forms grew out of crystallization conditions: monoclinic P21 and cubic I4132. The monoclinic crystal form was used to solve the structure of wild-type native LF, with zinc bound in the active site, to 2.2 Å resolution. The cubic crystal form (Figure 2) was instrumental in the introduction of the target peptide into the active site of LF through peptide solution soaks, thus leading to the crystal structure of the LF-MAPKK2 N-terminus peptide complex, to 3.9 Å resolution. All of the data for the cubic LF crystals and peptide complex were collected at the Stanford Synchrotron Radiation Laboratory (SSRL), mainly on Beamlines 7-1 and 9-1. Due to the large unit cell and weak diffraction properties of this crystal form, the use of the SSRL synchrotron source was essential to obtaining high-resolution data. Data were collected at SRS, Daresbury, ESRF, Grenoble, APS, Chicago and National Synchrotron Light Source, Brookhaven to solve the atomic resolution structures of PA, LF and EF.
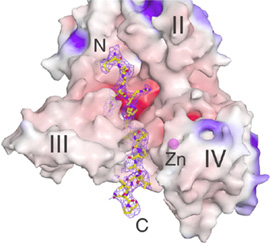
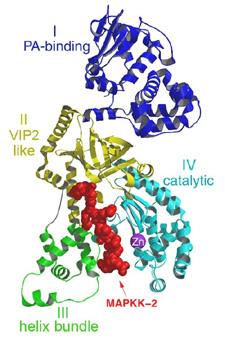
Lethal Factor (LF) comprises 4 structural domains: Domain I provide the binding surface for PA. Domain II resembles a toxin enzyme, VIP2, from Bacillus cereus, although it does not share its catalytic activity. Domain III is inserted into domain II, and has arisen from the duplication of a structural element from domain II. Domain IV, which resembles Domain I, is the catalytic center, with its protein fold resembling the protein family of the zinc-dependent proteinase typified by Thermolysin. Domains II, III and IV together create a groove - 40 Å long - into which a 16 -amino acid long peptide representing the N- terminus of its natural substrate MAPKK-2 binds (Figures 3 and 4). Thus, LF is an interesting example in protein evolution, in which the functional unit arises from gene duplication and fusion of domain building blocks. The structure reveals, for the first time, a protease bound to its uncleaved substrate. The shape of the active site groove allows for specificity but yet gives the freedom for variable target processing. The binding groove in LF is acidic in nature (8), and nicely complements the basic nature of the N-termini of the six MAPKKs recognized by LF (5), although higher resolution complexes will be necessary to explore these interactions in atomic detail. Because LF is able to cripple normal host cell functions through several targets all at once, its contributions to the seriousness of Anthrax infections is tremendous, and the key to LF efficiency lies primarily in the active center. Thus, understanding the crystal structure of LF, and atomic interactions with natural targets in its active center, will be crucial in the design of anti-toxins that inhibit LF activity.
- Leppla SH in Comprehensive Sourcebook of Bacterial Protein Toxins 2nd edn (eds Alouf JA & Freer J) 243-263 (Academic, London, 1999).
- Hanna PC, Acosta D & Collier RJ. On the role of macrophages in anthrax. Proc. Natl. Acad. Sci. USA 90, 10198-10201 (1993).
- Bradley KA, Mogridge J, Mourez M, Collier RJ & Young JAT. Identification of the cellular receptor for anthrax toxin. Nature 414, 225-229 (2001).
- Petosa C, Collier RJ, Klimpel KR, Leppla SH & Liddington RC. Crystal structure of the anthrax toxin protective antigen. Nature 385, 833-838 (1997).
- Vitale G, Bernadi L, Napolitani G, Mock M & Montecucco C. Susceptibility of mitogen-activated protein kinase kinase family members to proteolysis by anthrax lethal factor.Biochem. J. 352, 739-745 (2000).
- Duesbury NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson AK, Fukasawa K, Paull KD & Vande Woude GF. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280, 734-737 (1998).
- Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79, 3162-3166 (1982).
- Pannifer AD, Wong TY, Schwarzenbacher R, Renatus M, Petosa C, Bienkowska J, Lacy DB, Collier RJ, Park S, Leppla SH, Hanna P & Liddington RC. Crystal structure of the anthrax lethal factor. Nature 414, 229-233 (2001).
- Drum CL, Yan S-Z, Bard J, Shen Y-Q, Lu D, Soelaiman S, Grabarek Z, Bohm A & Tang W-J. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature415, 396-402 (2002).
- Kraulis PJ. Molscript - a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallog. 24, 946-950 ( 1991).
- Bacon DJ & Anderson WF. A fast algorithm for rendering space- filling molecule pictures. J. Mol. Graphics 6, 219-220 (1998).
- Merrit EA & Murphy MEP. Raster3D version 2.0. A program for photorealistic molecular graphics. Acta Crystallogr. D50, 869-873 (1994).
- Christopher JA. SPOCK: The Structural Properties Observation and Calculation Kit [program manual] (The Center for Macromolecular Design, Texas A&M University, College Station, TX, 1998).




