
The famous 17th-century Swedish warship Vasa has been on display in the Vasa Museum since 1990 (Figure 1). The Vasa sank on its maiden voyage in 1628, and was recovered in 1961 after 333 years in the cold brackish water of Stockholm harbor. After extensive conservation treatment, the oaken Vasa appeared in good condition (1). However, high acidity and a rapid spread of sulfate salts and elemental sulfur were recently observed on many wooden surfaces. A research team led by Prof. Magnus Sandström, University of Stockholm, have approached the problem by using X-ray absorption near edge spectroscopy (XANES) at the sulfur K-edge. The data were recorded on SSRL's Beam Line 6-2 (2) - for the first time on wooden core samples - to speciate the sulfur compounds (3,4). The XANES spectra, measured on samples extracted at various depth of the cores, unexpectedly revealed large amounts of embedded elemental sulfur (0.2-4 mass%) in the wood, also sulfate and, in minor amounts, several sulfur compounds of intermediate oxidation states (Figure 2). X-ray photoelectron spectroscopy (XPS; (5) was in addition used to quantify all elements at the same depths (cf. Figure 2,3). The study shows that in humid museum atmospheres a stepwise sulfur oxidation produces sulfuric acid:
S(s) + 3/2O2 + H2O ® 2H+(aq) + SO42- (1)
This oxidation is catalyzed by iron species in the wood (Figure 3) released from the completely corroded original iron bolts, as well as from those inserted after salvage. The overall quantity of elemental sulfur remaining in the Vasa is enough to produce more than 5000 kg of sulfuric acid when fully oxidized. Acidic wood hydrolysis is a severe threat to the continued preservation of the Vasa, and pH-raising treatments must be applied to arrest wood deterioration. There are similar problems for other wooden marine-archaeological artifacts (6). The team has also analyzed fresh cores from the submerged wreck of the Swedish warship Kronan (126 guns, sunk in battle in 1676 outside Öland in the Baltic), which contain a high amount of elemental sulfur and less sulfate. Cores from the Dutch Batavia, wrecked outside Western Australia in 1629, were found to contain mostly elemental sulfur and sulfate but also iron sulfides in wood with high iron content. Thus, the sulfur problem seems to be general and serious for waterlogged marine-archaeological wood recovered from anoxic conditions.
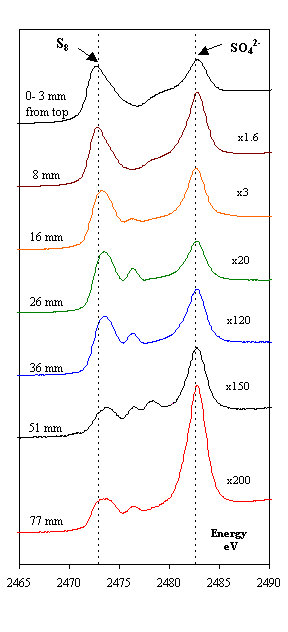
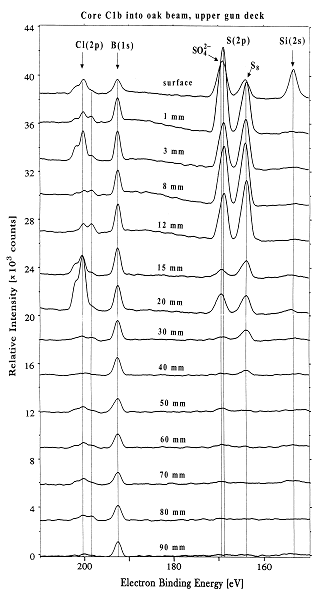
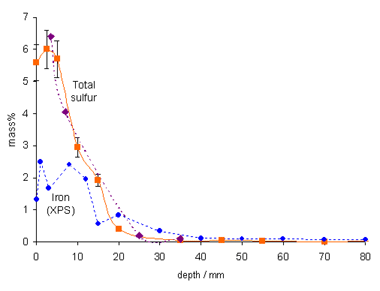
traces of pyrite FeS2. Reproduced by permission, (http://www.nature.com/).
Occasionally, the natural degradation processes have been arrested in marine burial that deposits wood in near anaerobic environment inhospitable to decay fungi and wood-borers, but with high content of hydrogen sulfide produced by sulfate-reducing bacteria metabolizing organic material. The analyses in this study indicate that when hydrogen sulfide in aqueous solution penetrates submerged waterlogged wood, it transforms and accumulates in the wood mainly as elemental sulfur. Whether this transformation is effectuated by sulfide-oxidizing bacteria (7) or is chemically controlled, is not yet clear. However, crystalline a-sulfur containing S8 crowns was found on and close below the wooden surfaces through identification by X-ray powder diffraction (XRD).
The amount of sulfate found in theVasa indicates that a substantial amount of the infused sulfur already has been oxidized to acid. This is consistent with the observation that during conservation, several tons of borax had to be added to neutralize the acid released from the wood into the recirculating polyethene glycol solutions (used for spray treatment to prevent wood shrinkage when drying), to keep the pH neutral, around 7 (1). In order to slow down the present oxidation process, information on the mechanism, rate-limiting factors, stability of the intermediates, dependence on catalysts, and microbial activity, will be essential.
Conservation procedures must be developed to remove the elemental sulfur from waterlogged wood, monitored by sulfur spectroscopy using synchrotron radiation. Otherwise, Nature's sulfidic preservation agent will give a very acidic aftertaste, and become a severe threat to continued preservation of precious artifacts in the museum atmosphere.
- Håfors, B. Conservation of the Swedish Warship Vasa from 1628, 1-180 (Vasa Museum, Stockholm, Sweden, 2001).
- Hedman, B., Frank, P., Penner-Hahn, J. E., Roe, A. L., Hodgson, K. O., Carlson, R. M. K., Brown, G., Cerino, J., Hettel, R., Troxel, T., Winick, H., Yang, J. Sulfur K-Edge X-Ray Absorption Studies Using the 54-Pole Wiggler at SSRL in Undulator Mode. Nucl. Instr. and Meth. A246, 797-800 (1986)
- Sandström, M., Jalilehvand, F., Persson, I., Gelius, U., Frank, P., Hall-Roth, I., Deterioration of the 17th-century warship Vasa by internal formation of sulphuric acid, Nature 415, 893-897 (2002) .
- Sandström, M., Jalilehvand, F., Persson, I., Gelius, U., Frank, P. Acidity and Salt Precipitation on the Vasa; The Sulfur Problem. In Proceedings of the 8th ICOM-CC WOAM Conference (ed. Hoffmann, P.) (ICOM, Committee for Conservation, Working Group on Wet Organic Archaeological Materials, Stockholm 2001). In press.
- Gelius, U., Wannberg, B., Baltzer, P., Fellner-Feldegg, H., Carlsson, G., Johansson, C.-G., Larsson, J., Münger, P., Vegerfors, G. A New ESCA Instrument with Improved Surface Sensitivity, Fast Imaging Properties and Excellent Energy Resolution. J. Electr. Spectr. Rel. Phenom. 52, 747-785 (1990).
- Information about historical ships can be found at the following World-Wide Web sites: for the Vasa, http://www.vasamuseet.se/indexeng.html and links therein; for the wreck ofKronan, http://www.kalmarlansmuseum.se/kronan/english/index.html; for the wreck of Batavia, http://www.mm.wa.gov.au/Museum/march/department/batavia.html.
- Pickering, I. J., George, G. N., Yu, E. Y., Brune, D. C. Tuschak, C., Overmann, J., Beatty, J. T., Prince, R. C. Analysis of Sulfur Biochemistry of Sulfur Bacteria Using X-ray Absorption Spectroscopy, Biochemistry 40, 8138-8145 (2001).
This work has been referenced in the following articles:
- Jim Gillon's News and Views article in Nature 415, 847 (2002)
- Dawn Levy's article in the Stanford Report (February 21, 2002)
- Donald McNeil's article in the New York Times (May 14, 2002)




