Axl is a receptor that belongs to the TAM family of receptor tyrosine kinases, which also includes Tyro3 and Mer. Axl has recently been shown to be an important oncology target, as signaling through the receptor drives metastasis, confers therapeutic resistance, and promotes disease progression across a myriad of human malignancies1,2. Furthermore, Axl overexpression on tumors has shown a strong correlation with disease stage and poor clinical prognosis, cementing its status as an important new target for therapeutic intervention. Despite this importance, relatively few Axl inhibitors have been developed to-date; these therapeutics for the most part comprise small molecule kinase inhibitors, which can exhibit off-target effects and dose limiting toxicities. The strong picomolar binding affinity between Axl and its ligand, growth arrest-specific 6 (Gas6), has made it challenging to develop biologics that can effectively inhibit this interaction3.
To address this issue, a group of researchers led by Jennifer Cochran and Amato Giaccia from Stanford University used protein engineering to generate a therapeutic that can effectively silence the Gas6-Axl signaling axis4. The engineered protein is a ‘receptor decoy’ that comprises the extracellular domains of the natural Axl receptor and is thereby capable of binding to and neutralizing the activity of Gas6. Using combinatorial protein engineering, mutations were randomly introduced in the Axl protein and variants with mutations that enhanced the affinity to Gas6 were identified by high-throughput screening approaches. The result of these engineering efforts was an Axl variant, MYD1, which bound to Gas6 with higher affinity than the natural Axl protein. This engineered decoy receptor displayed potent inhibition of metastatic disease in challenging pre-clinical models of breast and ovarian cancer4. It is noteworthy that the in vivo efficacy of the MYD1 inhibitor correlated with its affinity for the Gas6 target ligand.
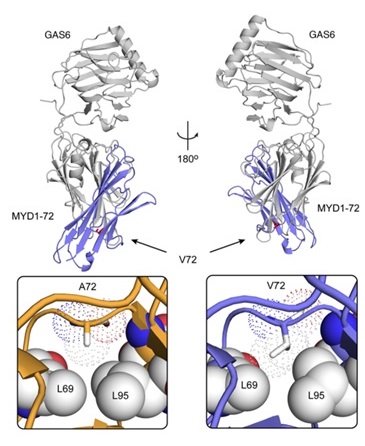
The current study, published in the Journal of Clinical Investigation, reports the successful development of a second-generation, high-affinity Axl decoy receptor with an apparent Gas6 affinity that is even tighter than MYD1. The new decoy receptor, MYD1-72, incorporated an additional amino acid mutation (A72V) identified through additional protein engineering efforts. This further evolved Axl variant profoundly inhibited disease progression in aggressive preclinical models of human cancers, and augmented the efficacy of chemotherapy in these studies. Importantly, to gain insights into the structural basis of the enhanced binding affinity, the crystal structure of MYD1-72 complexed with Gas6 was solved and compared to the structure of Gas6/MYD14. The diffraction data sets collected at BL12-2 revealed that the additional mutation resulted in a marked rearrangement of the amino acid side chains in the vicinity of residue 72, resulting in the gain of several favorable contacts (van der Waals as well as electrostatic interactions) and thereby providing important molecular level information that could explain the observed improvement in affinity. It is noteworthy that the poor diffraction quality and the variability in diffraction quality between crystals required screening of large number of crystals, a process that was feasible only by using the SSRL robot, highlighting its importance for overcoming experimental hurdles.
In summary, there is a critical need for the development and characterization of anti-Axl therapeutics for treating metastatic disease. However, the Axl inhibitors that have been generated so far have had limited success, primarily because of their comparatively poor binding affinity relative to the strength of the native Gas6/Axl interaction. The second-generation Axl decoy receptor reported here combined beneficial mutations of the first-generation inhibitor with an additional mutation to generate an inhibitor with a Gas6 binding affinity of less than 100 femtomolar, representing one of the tightest binding interactions found in nature. When directly compared with the most advanced anti-Axl small molecule inhibitors in the clinic, the new decoy receptor achieved superior antitumor efficacy while displaying no apparent toxicity in animal models of disease. The new receptor also showed improvements to the therapeutic index of current standard-of-care chemotherapies in preclinical models of advanced pancreatic and ovarian cancer. The elucidation of the structural basis for affinity enhancement lays the foundation for the production of more effective therapeutic proteins that leverage the advantageous characteristics of Axl-based decoy receptors.
- Y. Li et al., "Axl as a Potential Therapeutic Target in Cancer: Role of Axl in Tumor Growth, Metastasis and Angiogenesis", Oncogene 28, 3442 (2009).
- J. Hong, D. Peng, Z. Chen, V. Sehdev and A. Belkhiri, "ABL Regulation by AXL Promotes Cisplatin Resistance in Esophageal Cancer", Cancer Res. 73, 331 (2013).
- X. Ye et al., "An Anti-Axl Monoclonal Antibody Attenuates Xenograft Tumor Growth and Enhances the Effect of Multiple Anticancer Therapies", Oncogene 29, 5254 (2010).
- M. S. Kariolis et al., "An Engineered Axl ‘Decoy Receptor’ Effectively Silences the Gas6-Axl Signaling Axis", Nat. Chem. Biol. 10, 977 (2014).
M. S. Kariolis et al., “Inhibition of the GAS6/AXL Pathway Augments the Efficacy of Chemotherapies”, J. Clin. Invest. Nov. 29, 2016, DOI:10.1172/JCI85610.

