There has been a long-standing interest in blocking agents against influenza entry, such as inhibitors that can target the receptor binding site on the hemagglutinin surface glycoprotein (HA) to prevent viral attachment to host cells. Molecules have been designed based on the sialic acid receptor, although with very little success since sialic acid only has millimolar affinity to the HA. However, the recent discoveries and structural characterization of broadly neutralizing antibodies against the HA receptor binding site1 have revitalized interest in targeting the receptor pocket by antibody-based small molecules. In particular, antibody F045-092 was discovered to have neutralizing activity against divergent HA subtypes such as H3, H1, H2, and H5.2 The antibody binding site was mapped near the HA receptor binding site on the basis of escape mutant analysis. This remarkable breadth of recognition was unexpected as antibodies against the HA have long been considered to be strain-specific, especially at and around the highly variable membrane-distal globular head. Thus, a clear picture of the antibody’s epitope on the HA was of great interest.
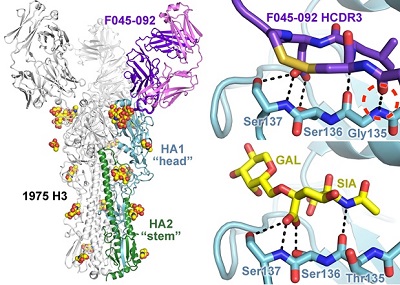
Figure 1. Recognition of the influenza hemagglutinin by antibody F045-092. The antibody inserts its long HCDR3 loop into the receptor binding site and mimics elements of the sialic acid receptor.
Using x-ray diffraction data collected from Beam Line 11-1 at SSRL, crystal structures of the F045-092 Fab and the A/Victoria/361/2011 (H3N2) HA were determined at 1.50 Å and 1.75 Å resolution, respectively. These structures revealed that the F045-092 antibody inserts a complementarity determining region (CDR) loop into the HA receptor binding site and uses receptor mimicry (Figure 1). Additional biophysical experimental data reveal that F045-092 recognizes a panel of divergent HA strains and subtypes, including all H3 avian and human isolates tested that range from 1963 to 2011. In addition, the antibody extends its breadth of recognition by using avidity through bivalent interactions of the IgG to overcome weak Fab interactions, which may aid in developing breadth. This feature is important since F045-092, as well as other receptor binding site-targeted antibodies, bind a larger footprint on the HA in comparison to the relatively small sialic acid receptor and inevitably contact variable residues outside of the binding pocket.
The combination of the structural details and binding mode of F045-092 and other receptor binding site-targeted antibodies has revealed recurring trends in recognition that can be utilized for structure-based drug discovery as well as vaccine design. Nearly all of the receptor binding site-targeted antibodies insert a hydrophobic residue into a conserved hydrophobic pocket of the HA that would be occupied by the acetamide moiety, and half of them use the side chain of aspartic acid in the CDR loop to overlap with the carboxylate moiety of sialic acid. Thus, structural studies of antibodies to the receptor binding site has given new insights both into broad neutralization of influenza virus as well as novel avenues to explore for vaccine design and small molecule discovery.
- P. S. Lee, I. A. Wilson, “Structural Characterization of Viral Epitopes Recognized by Broadly Cross-Reactive Antibodies”, Curr. Top. Microbiol. Immunol. (2014), doi: 10.1007/82_2014_413.
- N. Ohshima, Y. Iba, R. Kubota-Koketsu, Y. Asano, Y. Okuno, Y. Kurosawa, “Naturally Occurring Antibodies in Humans can Neutralize a Variety of Influenza Virus Strains, Including H3, H1, H2, and H5”, J. Virol. 85, 11048 (2011), doi: 10.1128/JVI.05397-11.
P. S. Lee, N. Ohshima, R. L. Stanfield, W. Yu, Y. Iba, Y. Okuno, Y. Kurosawa, I. A. Wilson, “Receptor Mimicry by Antibody F045-092 Facilitates Universal Binding to the H3 Subtype of Influenza Virus”, Nat. Commun. 5, 3614 (2014), doi: 10.1038/ncomms4614.




